Abstract
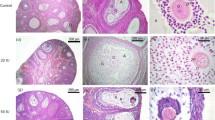
Obplacental giant cells are enlarged cells, found following implantation, in the antimesometrial region of the rabbit uterus. They probably originate from trophoblastic knobs that traverse the uterine epithelium during early implantation. Little is known about their function. In this study, trophoblast, placental, paraplacental and obplacental tissues at days 7–15 post-coitum, and enzyme-isolated giant cells at day 15 were studied by two-dimensional gel electrophoresis, followed by immunoblotting and light-microscopic immunohistochemistry, for the presence of human chorionic gonadotropin-like proteins. Immunostaining was performed by using anti-human chorionic gonadotropin antibodies. In gel electrophoresis of obplacental tissue and isolated giant cells, two proteins of human chorionic gonadotropin-like antigenicity at 26 kDa with pIs equivalent to pH 6.4 and 6.6 were found; they were absent in the placenta, paraplacenta, day-7 blastocyst and day-8 trophoblast. The onset of synthesis of these proteins could be observed when day-8 trophoblastic tissue was cultured in vitro for 24 h. In immunohistochemistry, only the obplacental giant cells showed a positive reaction, indicating that the production of chorionic gonadotropin occurs in this cell type.
Similar content being viewed by others
References
Bahl OP, Carlsen RB, Bellisario R, Swaminathan N (1972) Human chorionic gonadotropin, amino acid sequence of the α and β subunits Biochem Biophys Res Commun 48:416–420
Bill II CH, Keyes PL (1983) 17β-Estradiol maintains normal function of corpora lutea throughout pseudopregnancy in hypophysectomized rabbits. Biol Reprod 28:608–617
Blackburn DG, Osteen KG, Winfrey VP, Hoffman LH (1989) Obplacental giant cells of the domestic rabbit: development, morphology, and intermediate filament composition. J Morphol 202:185–203
Blum H, Beier H, Gross HJ (1987) Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93–99
Böving BG (1962) Anatomical analysis of rabbit trophoblast invasion. Contrib Embryol 37:33–55
Browning JY, Wolf RC (1981) Maternal recognition of pregnancy in the rabbit: effect of conceptus removal. Biol Reprod 24:293–297
Chiboka O, Casida LE, First NL (1977) Role of rabbit fetuses and placentas in the maintenance of gestation and parturition. J Anim Sci 46:776–783
Dannhorn DR, Gierhake S, Kirchner C (1991) Uteroglobin in the developing rabbit conceptus in vivo and in vitro. Anat Embryol 184:141–152
Dharmarajan AM, Zanagnolo VL, Dasko LM, Zirkin BR, Ewing LL, Wallach EE (1991) Estradiol regulation of the rabbit corpus luteum: in vivo and in vitro studies. Endocrinology 128:2678–2684
Enders AC, Schlafke S (1971) Penetration of the uterine epithelium during implantation in the rabbit. Am J Anat 132:219–240
Gadsby JE, Keyes PL (1984) Control of corpus luteum function in the pregnant rabbit: role of the placenta “placental luteotropin” in regulating responsiveness of corpora lutea to estrogen. Biol Reprod 31:16–24
Gadsby JE, Lancaster ML (1989) Rabbit placental-conditioned medium stimulates progesterone accumulation by granulosalutein cells in culture: preliminary characterization of a placental luteotropic hormone. Biol Reprod 40:239–249
Gadsby JE, Keyes PL, Bill CH (1983) Control of corpus luteum function in the pregnant rabbit: role of estrogen and lack of a direct luteotrophic role of the placenta. Endocrinology 113:2255–2262
Gründker C, Hrabé de Angelis M, Kirchner C (1993) Placental lactogen-like proteins in the rabbit placenta. Anat Embryol 188:395–399
Hoyer PB, Keyes PL, Niswender GD (1986) Size distribution and hormonal responsiveness of dispersed rabbit luteal cells during pseudopregnancy. Biol Reprod 34:905–910
Khyse-Anderson J (1984) Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose J Biochem Biophys Methods 10:203–209
Larsen JF (1963) Histology and fine structure of the avascular and vascular yolk sac placentae and obplacental giant cells in the rabbit. Am J Anat 112:269–283
Laskey RA, Mills AD (1975) Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem 56:335–341
Marcinkiewicz JL, Bahr JM (1993) Identification and preliminary characterization of luteotropic activity in the rabbit placenta. Biol Reprod 48:403–408
McLean MP, Miller JB (1985) Steroidogenic effect of 17β-estradiol on rabbit luteal cells in vitro: estrogen-induced maintenance of progesterone production. Biol Reprod 33:459–469
Meyer JM (1972) Action lutéotrophique des cellules géantes de l'untérus gravide chez la lapine. C R Acad Sci III 275:577–578
Meyer JM (1973) Cellules géantes trophoblastiques et corps jaunes chez la lapine. Arch Anat Microsc 62:151–172
Minot CS (1889) Uterus and embryo: 1. rabbit; 2. man. J Morphol 2:341–462
O'Farrel PH (1975) High resolution two-dimensional electrophoresis of proteins. J Biol Chem 250:4007–4021
Ogren L, Talamantes F (1988) Prolactins of pregnancy and their cellular source. Int Rev Cytol 112:1–65
Osteen K, Blackburn DG, Hoffmann LH (1987) Luteotropic effect of rabbit obplacental giant cells demonstrated in vitro. Biol Reprod 36 [Suppl 1]:140
Sherman MI (1983) Endocrinology of rodent trophoblast cells. In: Loke YW, Whyte A (eds) Biology of trophoblast. Elsevier, Amsterdam, pp 401–467
Soares MJ, Julian JA, Glasser SR (1985) Trophoblast giant cell release of placental lactogens: temporal and regional characteristics. Dev Biol 107:520–526
Swaminathan N, Bahl OP (1970) Dissociation and recombination of the subunits of human chorionic gonadotropin. Biochem Biophys Res Commun 40:422–427
Tovey ER, Baldo BA (1987) Comparison of semi-dry and conventional tank-buffer electrotransfer of proteins from polyacrylamide gels to nitrocellulose membranes. Electrophoresis 8:384–387
Vlasselaer P van, Vandeputte M (1984) Immunosuppressive properties of murine trophoblast. Cell Immunol 83:422–432
Weeransinghe KM, Gadsby JE (1990) Further studies on the isolation and characterization of a rabbit placental “luteotrophin”. Biol Reprod 42 [Suppl 1]: abstract 291
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gründker, C., de Angelis, M.H. & Kirchner, C. Chorionic gonadotropin-like proteins in the obplacental giant cells of the rabbit. Cell Tissue Res 278, 573–578 (1994). https://doi.org/10.1007/BF00331376
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00331376