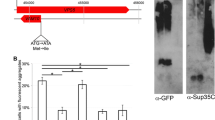
Abstract
A new approach involving the comparative analysis of proteins of crude cell lysate pellets from isogenic strains of Saccharomyces cerevisiae distinguished by their prion composition permitted us to identify a large group of prion-associated proteins in yeast cells. 35 proteins whose aggregation state depends on prion content have been identified by 2D-electrophoresis followed by the MALDI analysis of a recipient [psi −] strain and of [PSI +] cytoductant. Approximately half of these proteins belong to functional groups of chaperones and enzymes involved in glucose metabolism. Other proteins are involved in translation, stress response and protein degradation. The data obtained are compared with the results of other groups who used different approaches to detect proteins involved in prion aggregates.
Similar content being viewed by others
References
Baci, L., Bertini, I., Boca, M., Girotto, S., Martinelli, M., Valentine, J.S., and Vieru, M., SOD1 and Amyotrophic Lateral Sclerosis: Mutations and Oligomerization. PLoS ONE, 2008, vol. 3, pp. e1677.
Bagriantsev, S.N., Gracheva, E.O., Richmond, J.E., and Liebman, S.W., Variant-Specific [PSI+] Infection Is Transmitted by Sup35 Polymers within [PSI +] Aggregates with Heterogeneous Protein Composition. Mol. Biol. Cell., 2008, vol. 19, pp. 2433–2443.
Buchan, J.R., Muhlrad, D., and Parker, R., P. Bodies Promote Stress Granule Assembly in Saccharomyces cerevisiae. J. Cell. Biol., 2008, vol. 183, pp. 441–455.
Cashikar, A.G., Duennwald, M., and Lindquist, S.L., A Chaperone Pathway in Protein Disaggregation: HSP26 Alters the Nature of Protein Aggregates to Facilitate Reactivation by HSP104. J. Biol. Chem., 2005, vol. 280, pp. 23869–23875.
Chernoff, Y.O., Cellular Control of Prion Formation and Propagation in Yeast, in Prions and Prion Diseases: Current Perspectives, Wymondham: Horizon Bioscience, 2004, pp. 257–303.
Chernoff, Y.O., Identity Determinants of Infectious Proteins. Proc. Natl. Acad. Sci. USA, 2008, vol. 105, pp. 13191–13192.
Chernoff, Y.O., Lindquist, S.L., Ono, B., Inge-Vechtomov, S.G., and Liebman, S.W., Role of the Chaperone Protein hsp104 in Propagation of the Yeast Prion-Like Factor [PSI +]. Science, 1995, vol. 268, pp. 880–884.
Chernoff, Y.O., Newnam, G.P., Kumar, J., Allen, K., and Zink, A.D., Evidence of a Protein Mutator in Yeast: Role of the Hsp70-Related Chaperone Ssb in Formation, Stability, and Toxicity of the [PSI] Prion. Mol. Cell. Biol., 1999, vol. 19, pp. 8103–8112.
Chernoff, Y.O., Uptain, S.M., and Lindquist, S.L., Analysis of Prion Factors in Yeast. Meth. Enzymol., 2002, vol. 351, pp. 499–537.
Chernova, T.A., Allen, K.D., Wesoloski, L.M., Shanks, J.R., Chernoff, Y.O., and Wilkinson, K.D., Pleiotropic Effects of Ubp6 Loss on Drug Sensitivities and Yeast Prion Are Due to Depletion of the Free Ubiquitin Pool. J. Biol. Chem., 2003, vol. 278, pp. 52102–52115.
Cox, B.S., Byrne, L., and Tuite, M.F., Prion Stability, in Protein-Based Inheritance, Austin, Texas: Landes Bioscience, 2007, pp. 56–72.
Derkatch, I.L. and Liebman, S.W., Prion-Prion Interactions, in Protein-Based Inheritance, Austin, Texas: Landes Bioscience, 2007, pp. 39–55.
Du, Z., Park, K.-W., Yu, H., Fan, Q., and Li, L., Newly Identified Prion Linked to the Chromatin-Remodeling Factor Swi1 in Saccharomyces cerevisiae, Nature Genetics, 2008, vol. 40, pp. 460–465.
Elam, J.S., Taylor, A.B., Strange, R., Antonyuk, S., Doucette, P.A., Rodriguez, J.A., Hasnain, S.S., Hayward, L.J., Valentine, J.S., Yeates, T.O., and Hart, P.J., Amyloid-Like Filaments and Water-Filled Nanotubes Formed by SOD1 Mutant Proteins Linked to Familial ALS. Nat. Struct. Biol., 2003, vol. 10, pp. 461–467.
Erjavec, N., Larsson, L., Grantham, J., and Nystrom, T., Accelerated Aging and Failure to Segregate Damaged Proteins in Sir2 Mutants Can Be Suppressed by Overproducing the Protein Aggregation-Remodeling Factor Hsp104p. Genes Dev., 2007, vol. 21, pp. 2410–2421.
Glabe, C.G., Statistical Classification of Toxic Amyloid Oligomers. J. Biol. Chem., 2008, vol. 283, pp. 29639–29643.
Griffith, J.S., Self-Replication and Scrapie. Nature, 1967, vol. 215, pp. 1943–1044.
Harrison, L.B., Yu, Z., Stajich, J.E., Dietrich, F.S., and Harrison, P.M., Evolution of Budding Yeast Prion-Determinant Sequences across Diverse Fungi. J. Mol. Biol., 2007, vol. 368, pp. 273–282.
Herczenik, E. and Gebbink, M.F.B.G., Molecular and Cellular Aspects of Protein Misfolding and Disease. FASEB J., 2008, vol. 22, pp. 2115–2133.
Jones, G.W. and Masison, D.C.. Saccharomyces cerevisiae Hsp70 Mutations Affect [PSI+] Prion Propagation and Cell Growth Differently and Implicate Hsp40 and Tetratri-copeptide Repeat Cochaperones in Impairment of [PSI +]. Genetics, 2003, vol. 163, pp. 495–506.
Jung, G., Jones, G., Wegrzyn, R.D., and Masison, D.C., A Role for Cytosolic Hsp70 in Yeast [PSI+] Prion Propagation and [PSI+] as a Cellular Stress. Genetics, 2000, vol. 156, pp. 559–570.
Kryndushkin, D.S., Alexandrov, I.M., Ter-Avanesyan, M.D., and Kushnirov, V.V., Yeast [PSI+] Protein Aggregates Are Formed by Small Sup35 Polymers Fragmented by Hsp104. J. Biol. Chem., 2003, vol. 278, pp. 49636–49643.
Kushnirov, V.V., Alexandrov, I.M., Mitkevich, O.V., Shkundina, I.S., and Ter-Avanesyan, M.D., Purification and Analysis of Prion and Amyloid Aggregates. Methods, 2006, vol. 39, pp. 50–55.
Kushnirov, V.V., Vishnevskaya, A.B., Alexandrov, A.M., and Ter-Avanesyan, M.D., Prion and Nonprion Amyloids: A Comparison Inspired by the Yeast Sup35 Protein, in Protein-Based Inheritance, Austin, Texas: Landes Bioscience, 2007, pp. 73–82.
Laemmli, U.K., Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature, 1970, vol. 227, pp. 680–685.
Lian, H.Y., Jiang, Y., Zhang, H., Jones, G.W., and Perrett, S., The Yeast Prion Protein Ure2: Structure, Functions, and Folding. Biochim. Biophys. Acta, 2006, vol. 1764, pp. 535–545.
Lian, H.Y., Zhang, H., Zhang, Z.-R., Loovers, H.M., Jones, G.W., Rowling, P.J.E., Itzhaki, L.S., Zhou, J.-M., and Perrett, S., Hsp40 Interacts Directly with the Native State of the Yeast Prion Protein Ure2 and Inhibits Formation of Amyloid-Like Fibrils. J. Biol. Chem., 2007, vol. 282, pp. 11931–11940.
Nemecek, J., Nakayashiki, T., and Wickner, R.B., A Prion of Yeast Metacaspase Homolog (Mca1p) Detected by a Genetic Screen. Proc. Natl. Acad. Sci. USA, 2009, vol. 106, pp. 1892–1896.
Nevzglyadova, O.V., Artemov, A.V., Mittenberg, A.G., Mikhailova, E.V., Kuznetsova, I.M., Turoverov, K.K., and Soidla, T.R., Effect of the Red Pigment on Protein Amyloidization in Yeast. Tsitologiia, 2010a, vol. 51 (in press).
Nevzglyadova, O.V., Artemov, A.V., Mittenberg, A.G., Mikhailova, E.V., Kuznetsova, I.M., Turoverov, K.K., and Soidla, T.R., Yeast Protein Aggregates, Containing Chaperones and Glucose Metabolism Enzymes, in Molecular Chaperones: Roles, Structures and Mechanisms, Hauppauge, New York: Nova Publ., 2010b.
Nevzglyadova, O.V., Artyomov, A.V., Mikhailova, E.V., and Soidla, T.R., The Impact of Manipulations with Cytoplasmically Inherited Factors on Nuclear Transmission and Degradation in Yeast Heterokaryons. Curr. Genet., 2004, vol. 45, pp. 273–282.
Nevzglyadova, O.V., Kuznetsova, I.M., Artemov, A.V., Mikhailova, E.V., Turoverov, K.K., and Soidla, T.R., Estimating of Changes in the Amyloid and Prion Content of Yeast Cells. Tsitologiia, 2008, vol. 50 (1), pp. 40–48.
Patel, B.K., Gavin-Smyth J., and Liebman, S.W., The Yeast Global Transcriptional Co-repressor Protein Cyc8 Can Propagate as a Prion. Nature Cell. Biol., 2009, vol 11, pp. 344–349.
Prion Biology and Diseases, Prusiner, S.B., Ed., 2nd ed., Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 2004.
Protein-Based Inheritance, Chernoff, Y.O., Ed., Landes Bioscience: Austin, Texas, 2007.
Rikhvanov, E.G., Romanova, N.V., and Chernoff, Y.O., Chaperone Effects on Prion and Nonprion Aggregates, in Protein-Based Inheritance, Austin, Texas: Landes Bioscience, 2007, pp. 83–92.
Roberts, B.T. and Wickner, R.B., A Class of Prions That Propagate via Covalent Auto-activation. Genes Dev., 2003, vol. 17, pp. 2083–2087.
Satpute-Krishnan, P. and Serio, T.R., Prion Protein Remodeling Confers an Immediate Phenotype Switch. Nature, 2005, vol. 437, pp. 262–265.
Severin, S.E. and Solov’ev, G.A.. Praktikum po biokhimii (A Practical Course in Biochemistry), Moscow: Mosk. Gos. Univ., 1989.
Sharma, D., Stanley, R.F., and Masison, D.C., Curing of Yeast [URE3] Prion by the Hsp40 Cochaperone Ydj1p Is Mediated by Hsp70. Genetics, 2009, vol. 181, pp. 129–137.
Sherman, F., Fink, G.R., and Hicks, J.B.. Laboratory Course Manual for Methods in Yeast Genetics, Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press, 1986.
Sitia, R. and Molteni, S.N., Stress, Protein (Mis)folding, and Signaling: The Redox Connection. Sci. STKE, 2004, vol. 239, p. e27.
Takahashi, A., Hara, H., Kurahashi, H., and Nakamura, Y., A Systematic Evaluation of the Function of the Protein-Remodeling Factor Hsp104 in [PSI+] Prion Propagation in S. cerevisiae by Comprehensive Chromosomal Mutations. Prion, 2007, vol. 1, pp. 69–77.
Taneja, V., Maddelein, M.L., Talarek, N., Saupe, S.J., and Liebman, S.W., A Non-Q/N-Rich Prion Domain of a Foreign Prion, [Het-s], Can Propagate as a Prion in Yeast. Mol. Cell., 2007, vol. 27, pp. 67–77.
Towbin, H., Staehelin, T., and Gordon, J., Electrophoretic Transfer of Proteins from Polyacrylamide Gels to Nitrocellulose Sheets: Procedure and Some Applications. Proc. Natl. Acad. Sci. USA, 1979, vol. 76, pp. 4350–4354.
Tuite M.F. and Cox, B.S., The Genetic Control of the Formation and Propagation of the [PSI+] Prion in Yeast. Prion, 2007, vol. 1, pp. 101–109.
Uversky, V.N., Oldfield, C.J., and Dunker, A.K., Intrinsically Disordered Proteins in Human Diseases: Introducing the D2 Concept. Annu. Rev. Biophys., 2008, vol. 37, pp. 215–246.
Uversky, V.N., Amyloidogenesis of Natively Unfolded Proteins. Curr. Alzheimer Res., 2008, vol. 5, pp. 260–287.
Wang, Y., Meriin, A.B., Costello, C.E., and Sherman, M.Y., Characterization of Proteins Associated with Polyglutamine Aggregates: A Novel Approach towards Isolation of Aggregates from Protein Conformation Disorders. Prion, 2007, vol. 1, pp. 128–135.
Wang, Y., Meriin, A.B., Zaarur, N., Romanova, N.V., Chernoff, Y.O., Costello, C.E., and Sherman, M.Y., Abnormal Proteins Can Form Aggresome in Yeast: Aggresome-Targeting Signals and Components of the Machinery. FASEB J., 2008, vol. 23, pp. 451–463.
Watt, N.T., Taylor, D.R., Gillott, A., Thomas, D.A., Sumudhu, W., Perera, S., and Hooper, N.G., Reactive Oxygen Species-Mediated β-Cleavage of the Prion Protein in the Cellular Response to Oxidative Stress. J. Biol. Chem., 2005, vol. 280, pp. 35914–35921.
Wickner, R.B., [URE3] as an Altered URE2 Protein: Evidence for a Prion Analog in S. cerevisiae, Science, 1994, vol. 264, pp. 566–569.
Wickner, R.B., Edskes, H.K., and Shewmaker, F., How to Find a Prion: [URE3], [PSI +] and [beta]. Methods, 2006, vol. 39, pp. 3–8.
Wickner, R.B., Edskes, H.K., Shewmaker, F., Nakayashiki, T., Engel, A., McCann, L., and Kryndushkin, D., Yeast Prions: Evolution of the Prion Concept. Prion, 2007, vol. 1, pp. 94–100.
Wickner, R.B., Shewmaker, F., Kryndushkin, D., and Edskes, H.K., Protein Inheritance (Prions) Based on Parallel In-register β-Sheet Amyloid Structures. BioEssays, 2008, vol. 30, pp. 955–964.
Zakharov, I.A. and Yarovoy, B., Cytoduction as a New Tool in Studying the Cytoplasmic Heredity in Yeast. Mol. Cell. Biochem., 1977, vol. 14, pp. 15–18.
Zhou, R.Y., Li, X.L., Li, L.H., Li, X.Y., and Feng, F.J., Evolution and Differentiation of the Prion Protein Gene (PRNP) among Species. J. Heredity, 2008, vol. 99, pp. 647–652.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © O.V. Nevzglyadova, A.V. Artemov, A.G. Mittenberg, E.I. Kostyleva, E.V. Mikhailova, K.V. Solovyov, I.M. Kuznetsova, K.K. Turoverov, T.R. Soidla, 2010, published in Tsitologiya, Vol. 52, No. 1, 2010, pp. 63–79.
Rights and permissions
About this article
Cite this article
Nevzglyadova, O.V., Artemov, A.V., Mittenberg, A.G. et al. Comparison of crude lysate pellets from isogenic strains of yeast with different prion composition: Identification of prion-associated proteins. Cell Tiss. Biol. 4, 36–53 (2010). https://doi.org/10.1134/S1990519X10010049
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X10010049