Abstract
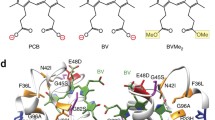
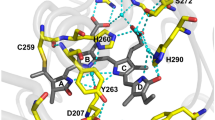
Fluorescent proteins (FPs) are widely used as genetically encoded markers for quantitative and noninvasive study of biological processes. Development of biomarkers that are fluorescent in the near-infrared spectral range allows the tissues of animals to be studied at a deeper level because they are more permeable to the light of this wavelength range than that of visible range. Such properties as low molecular weight and monomeric state are important for widespread use of FPs. In this paper, we managed to obtain FP based on the chromophore-binding domain of bacterial phytochrome (BphP) from Rhodopseudomonas palustris (RpB-phP1), named GAF-FP, with a molecular weight of ~19 kDa, which is half that of other FP based on BphP and 1.4 times lower than that of commonly used GFP-like proteins, which are fluorescent in the near-infrared range. In contrast to most other near-infrared FPs, GAF-FP is a monomer, which has a high photostability, and its structure is stable to the incorporation of small peptide inserts. Moreover, GAF-FP is capable of covalent attachment of two different tetrapyrrole chromophores: phycocyanobilin (PCB) and biliverdin (BV), which is contained in mammalian tissues. GAF-FP with attached BV as a chromophore (GAF-FP–BV) has the main absorption band with a maximum at 635 nm. The fluorescence maximum falls at 670 nm, whereby GAF-FP has a high ratio of the fluorescence signal to the background signal even if FP is localized at a depth of several mm below the tissue surface. Together with the near-infrared absorption band, GAF-FP–BV also has an absorption band in the violet region of the spectrum with a maximum at 378 nm. We used this property to design a chimeric protein consisting of modified luciferase from Renilla reniformis (RLuc8) and GAF-FP. We showed resonance energy transfer from the substrate, the excited state of which occurs when oxidized by luciferase, to the chromophore GAF-FP–BV in the designed fusion protein. In the absence of an energy acceptor, RLuc8 catalyzes the cleavage of the substrate with the emission of the light with a maximum at 400 nm. At the same time, the energy from the substrate is transferred to the FP chromophore and then emitted in the near-infrared range corresponding to the spectrum of GAF-FP fluorescence in the GAF-FP–RLuc8 chimeric protein. These results open the way for the development of new small near-infrared FPs based on various natural BphPs with a view to their widespread use in cell and molecular biology.
Similar content being viewed by others
Abbreviations
- BphP:
-
bacterial phytochrome
- BV:
-
biliverdin IXa
- PCB:
-
phycocyanobilin
- FP:
-
fluorescent protein
- GFP:
-
green fluorescent protein
- RLuc8:
-
Renilla reniformis luciferase
References
Auldridge, M.E., Satyshur, K.A., Anstrom, D.M, and Forest, K.T., Structure-guided engineering enhances a phytochrome- based infrared fluorescent protein, J. Biol. Chem., 2012, vol. 287, pp. 7000–7009.
Bellini, D. and Papiz, M.Z., Structure of a bacteriophytochrome and light-stimulated protomer swapping with a gene repressor, Structure, 2012, vol. 20, pp. 1436–1446.
Bhattacharya, S., Auldridge, M.E., Lehtivuori, H., Ihalainen, J.A., and Forest, K.T., Origins of fluorescence in evolved bacteriophytochromes, J. Biol. Chem., 2014, vol. 289, pp. 32144–32152.
Bhoo, S.H., Davis, S.J., Walker, J., Karniol, B., and Vierstra, R.D., Bacteriophytochromes are photochromic histidine kinases using a biliverdin chromophore, Nature, 2001, vol. 414, pp. 776–779.
Chen, M., Li, W., Zhang, Z., Liu, S., Zhang, X., Zhang, X.E., and Cui, Z., Novel near-infrared BiFC systems from a bacterial phytochrome for imaging protein interactions and drug evaluation under physiological conditions, Biomaterials, 2015, vol. 48, pp. 97–107.
Filonov, G.S., Piatkevich, K.D., Ting, L.M., Zhang, J., Kim, K., and Verkhusha, V.V., Bright and stable near-infrared fluorescent protein for in vivo imaging, Nat. Biotechnol., 2011, vol. 29, pp. 757–761.
Filonov, G.S. and Verkhusha, V.V., A near-infrared bifc reporter for in vivo imaging of protein-protein interactions, Chem. Biol., 2013, vol. 20, pp. 1078–1086.
Fischer, A.J., Rockwell, N.C., Jang, A.Y., Ernst, L.A., Waggoner, A.S., Duan, Y., Lei, H., and Lagarias, J.C., Multiple roles of a conserved GAF domain tyrosine residue in cyanobacterial and plant phytochromes, Biochemistry, 2005, vol. 44, pp. 15203–15215.
Gambetta, G.A. and Lagarias, J.C., Genetic engineering of phytochrome biosynthesis in bacteria, Proc. Natl. Acad. Sci. U. S. A., 2001, vol. 98, pp. 10566–10571.
Ishizuka, T., Narikawa, R., Kohchi, T., Katayama, M., and Ikeuchi, M., Cyanobacteriochrome TePixJ of Thermosyn-echococcus elongatus harbors phycoviolobilin as a chromophore, Plant Cell Physiol., 2007, vol. 48, pp. 1385–1390.
Lamparter, T., Evolution of cyanobacterial and plant phytochromes, FEBS Lett., 2004, vol. 573, pp. 1–5.
Narikawa, R., Fukushima, Y., Ishizuka, T., Itoh, S., and Ikeuchi, M., A novel photoactive GAF domain of cyanobacteriochrome AnPixJ that shows reversible green/red photoconversion, J. Mol. Biol., 2008, vol. 380, pp. 844–855.
Narikawa, R., Nakajima, T., Aono, Y., Fushimi, K., Enomoto, G., Ni, W., Itoh, S., Sato, M., and Ikeuchi, M., A biliverdin-binding cyanobacteriochrome from the chlorophyll D-bearing cyanobacterium Acaryochloris marina, Sci. Rep., 2015, vol. 5, p. 7950.
Pedelacq, J.D., Cabantous, S., Tran, T., Terwilliger, T.C., and Waldo, G.S., Engineering and characterization of a superfolder green fluorescent protein, Nat. Biotechnol., 2006, vol. 24, pp. 79–88.
Piatkevich, K.D., Subach, F.V., and Verkhusha, V.V., Engineering of bacterial phytochromes for near-infrared imaging, sensing, and light-control in mammals, Chem. Soc. Rev., 2013a, vol. 42, pp. 3441–3452.
Piatkevich, K.D., Subach, F.V., and Verkhusha, V.V., Farred light photoactivatable near-infrared fluorescent proteins engineered from a bacterial phytochrome, Nat. Commun., 2013b, vol. 4, p. 2153.
Rockwell, N.C. and Lagarias, J.C., A brief history of phytochromes, Eur. J. Chem. Phys. Phys. Chem., 2010, vol. 11, pp. 1172–1180.
Rockwell, N.C., Martin, S.S., and Lagarias, J.C., Red/green cyanobacteriochromes: sensors of color and power, Biochemistry, 2012, vol. 51, pp. 9667–9677.
Saito, K., Chang, Y.F., Horikawa, K., Hatsugai, N., Higuchi, Y., Hashida, M., Yoshida, Y., Matsuda, T., Arai, Y., and Nagai, T., Luminescent proteins for high-speed singlecell and whole-body imaging, Nat. Commun., 2012, vol. 3, pp. 1262.
Schneider, C.A., Rasband, W.S., and Eliceiri, K.W., NIH image to ImageJ: 25 years of image analysis, Nat. Methods, 2012, vol. 9, pp. 671–675.
Shcherbakova, D.M. and Verkhusha, V.V., Near-infrared fluorescent proteins for multicolor in vivo imaging, Nat. Methods, 2013, vol. 10, pp. 751–754.
Shcherbakova, D.M., Shemetov, A.A., Kaberniuk, A.A., and Verkhusha, V.V., Natural photoreceptors as a source of fluorescent proteins, biosensors, and optogenetic tools, Ann. Rev. Biochem., 2015, vol. 84, pp. 519–550.
Shu, X., Royant, A., Lin, M.Z., Aguilera, T.A., Lev-Ram, V., Steinbach, P.A., and Tsien, R.Y., Mammalian expression of infrared fluorescent proteins engineered from a bacterial phytochrome, Science, 2009, vol. 324, pp. 804–807.
Stepanenko, O.V., Bublikov, G.S., Stepanenko, O.V., Shcherbakova, D.M., Verkhusha, V.V., Turoverov, K.K., and Kuznetsova, I.M., A knot in the protein structure— probing the near-infrared fluorescent protein iRFP designed from a bacterial phytochrome, FEBS J., 2014, vol. 281, pp. 2284–2298.
Takai, A., Nakano, M., Saito, K., Haruno, R., Watanabe, T.M., Ohyanagi, T., Jin, T., Okada, Y., and Nagai, T., Expanded palette of nano-lanterns for real-time multicolor luminescence imaging, Proc. Natl. Acad. Sci. USA, 2015, vol. 112, pp. 4352–4356.
Toh, K.C., Stojkovic, E.A., van Stokkum, I.H., Moffat, K., and Kennis, J.T., Fluorescence quantum yield and photochemistry of bacteriophytochrome constructs, Phys. Chem. Chem. Phys., 2011, vol. 13, pp. 11985–11997.
Wagner, J.R., Zhang, J., Brunzelle, J.S., Vierstra, R.D., and Forest, K.T., High resolution structure of Deinococcus bacteriophytochrome yields new insights into phytochrome architecture and evolution, J. Biol. Chem., 2007, vol. 282, pp. 12298–12309.
Yu, D., Gustafson, W.C., Han, C., Lafaye, C., Noirclerc-Savoye, M., Ge, W.P., Thayer, D.A., Huang, H., Kornberg, T.B., Royant, A., Jan, L.Y., Jan, Y.N., Weiss, W.A., and Shu, X., An improved monomeric infrared fluorescent protein for neuronal and tumour brain imaging, Nat. Commun., 2014, vol. 5, pp. 3626.
Zhang, J., Wu, X.J., Wang, Z.B., Chen, Y., Wang, X., Zhou, M., Scheer, H., and Zhao, K.H., Fused-gene approach to photoswitchable and fluorescent biliproteins, Angew. Chem., 2010, vol. 49, pp. 5456–5458.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © K.A. Rumyantsev, D.M. Shcherbakova, N.I. Zakharova, V.V. Verkhusha, K.K. Turoverov, 2016, published in Tsitologiya, 2016, Vol. 58, No. 10, pp. 744–754.
Rights and permissions
About this article
Cite this article
Rumyantsev, K.A., Shcherbakova, D.M., Zakharova, N.I. et al. Design of near-infrared single-domain fluorescent protein GAF-FP based on bacterial phytochrome. Cell Tiss. Biol. 11, 16–26 (2017). https://doi.org/10.1134/S1990519X17010102
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X17010102