Abstract
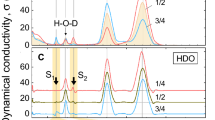
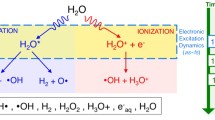
The ionization constant of water Kw (or pH) is currently determined on the proton conductivity σ0 which is measured at frequencies lower than 107 Hz. We develop the idea that the high frequency conductivity σ∞ (~1011 Hz), rather than σ0 represents a net proton dynamics in water. We count the concentration c of the H3O+ and OH– ions from σ∞ to find c to be not dependent on temperature. We conclude that spontaneous ionization of H2O molecules is not essential in water electrodynamics; the common Kw reflects the thermoactivation of the H3O+ and OH– ions from the potential of their interaction; the lifetime of a target water molecule does not exceed parts of nanosecond.
Similar content being viewed by others
References
D. Eisenberg and W. Kauzman, The Structure and Properties of Water (Clarendon Press, Oxford, UK, 1969).
P. L. Geissler, C. Dellago, D. Chandler, J. Hutter, and M. Parrinello, Science 291, 2121–2124 (2001).
D. Marx, Chem. Phys. Chem. 7, 1849 (2006).
Glasstone S., An Introduction to Electrochemistry, 4th edition (East West Press PVT. LTD, New Delhi, India, 1974).
J. O’ M. Bockris and A. K. N. Reddy, Modern Electrochemistry 1: Ionics, 2nd edition (Kluwer Academic Publishers, New York, 1998).
R. G. Bates, Determination of p H. Theory and Practice (John Wiley & Sons Inc. NY, London, Sydney, 1964).
A. V. Bandura and S. N. Lvov, J. Phys. Chem. Ref. Data 35 (1), 15–30 (2006).
T. Yagasaki, K. Iwahashi, S. Saito, and I. Ohmine, J. Chemical Physics 122, 144504 (2005).
A. A. Volkov, V. G. Artemov, and A. V. Pronin, EPL 106, 46004 (2014).
J. Rajeev, Physical Chemistry, Electrochemistry I (School of Studies in Chemistry, Jiwaji University, Gwalior–11, 2015), wwwacademiaedu.
T. S. Light and S. L. Licht, Anal. Chem. 59, 2327 (1987).
V. Scherbakov, Y. Artemkina, V. Ermakov, Electrolyte Solutions (Palmarium Acad. Publishing, Saarbrucken, 2012), p. 132 [in Russian].
CRC Handbook of Chemistry, and Physics, 70th Edition, Ed. By R. C. Weast (CRC Press, Boca Raton, FL, 1989), D-221.
V. G. Artemov, A. A. Volkov, N. N. Sysoev, and A. A. Volkov, EPL 109, 26002 (2015).
S. Meiboom, J. Chem. Phys. 34, 375 (1961).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.G. Artemov, A.A. Volkov, Jr., N.N. Sysoev, A.A. Volkov, 2016, published in Doklady Akademii Nauk, 2016, Vol. 466, No. 2, pp. 154–157.
Rights and permissions
About this article
Cite this article
Artemov, V.G., Volkov, A.A., Sysoev, N.N. et al. On autoionization and pH of liquid water. Dokl. Phys. 61, 1–4 (2016). https://doi.org/10.1134/S1028335816010043
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1028335816010043