Abstract
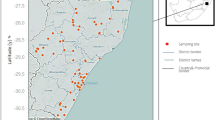
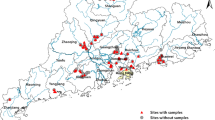
In Lake Albert, an ecological study was conducted, between June 2000 and May 2003, which assessed snail population dynamics, parasite infection patterns and interplay of environmental factors upon Biomphalaria. Monthly sampling surveys were conducted at 29 sites monitoring populations of Biomphalaria stanleyi and Biomphalaria sudanica. Altogether, a total of 21,715 B. stanleyi and 8452 B. sudanica were collected during the period. Both species could be found infected with Schistosoma mansoni although infection prevalence was significantly higher (p < 0.01**) in B. stanleyi (4.4%) than in B. sudanica (3.5%). Each species occupied slightly different aquatic niches with B. stanleyi preferring deeper water habitats whilst B. sudanica was found along the shoreline in shallower water. B. stanleyi was more widely distributed among the sampling locations (19 sites) than B. sudanica (10 sites). Of the four villages included in the study area, snails from sites near Piida and Bugoigo villages had the highest schistosome infection rates, presumably attributable to the closer proximity of people with intestinal schistosomiasis. After inspection of cross-correlation plots which identified most suitable time lags, snail density dynamics could be associated with seasonal variations inclusive of: air temperature, rainfall, lake level, water temperature, water conductivity and water pH. These temporal observations better reveal the relationship between snail populations and environmental factors, providing important information concerning the relative roles of B. stanleyi and B. sudanica in transmission of S. mansoni and development of integrated strategies for disease control around Lake Albert.
Similar content being viewed by others
References
Babiker A., Blannkspoor H. D., Wassila W., Fenwick A. and Daffalla A.A. (1985). Transmission of Schistosoma haematobium in North Gezira, Sudan. Journal of Medicine and Hygiene 88: 65–73
Beauchamp R. S. A. (1956). The electrical conductivity of the headwaters of the White Nile. Nature 178: 616–619
Beadle, L. C., 1974. Lakes Albert and Rudolf. In Inland Waters of Tropical Africa: An Introduction to Tropical Limnology. Longman, London: 138–141
Booth M., Mwatha J. K., Joseph S., Jones F. M., Kadzo H., Kazibwe F., Kemijumbi J., Kariuki H. C., Kimani G., Ouma J. H., Kabatereine N. B., Vennervald B. J. and Dunne D. W. (2004). Periportal fibrosis in human Schistosoma mansoni infection is associated with low IL-10, low IFN-γ, high TNF-α, or low RANTES, depending on age and gender. The Journal of Immunology 172: 1295–1303
Bradley D. J., Sturrock R. F. and Williams P. N. (1967). The circumstantial epidemiology of Schistosoma haematobium in Lango district, Uganda. East African Medical Journal 44: 193–204
Brown D. S. (1994). Freshwater Snails of Africa and Their Medical Importance. Taylor & Francis, London, 609
Coura Filho P., Mendes N. M., Souza C. P. and Pereira J. P. (1992). The prolonged use of niclosamide as molluscicides for the control of Schistosoma mansoni. Revista do Instituto de Medinica Tropicale Sao Paulo 34: 427–431
Chandiwana S. K. (1986). How Schistosoma mansoni eggs reach water bodies. Transactions of the Royal Society of Tropical Medicine and Hygiene 80: 963–964
Chen M. G. (1999). Progress in schistosomiasis control in China. Chinese Medical Journal (Peking) 112: 930–933
Christie J. D. and Upatham E. S. (1977). Control of Schistosoma mansoni transmission by chemotherapy in St. Lucia. 11. Biological results. American Journal of Tropical Medicine and Hygiene 26: 894–898
Cridland C. C. (1955). The experimental infection of several species of African fresh water snails with Schistosoma mansoni and S. haematobium. The Journal of Tropical Medicine and Hygiene 58: 1–11
Danish Bilharziasis Laboratory, 1987. A Field Guide to African Freshwater Snails, (2nd edn.) 2. East African species. WHO Collaborating Centre, Copenhagen: 29–32
Dunne D. W., Vennervald B. J., Booth M., Joseph S., Fitzsimmons C. M., Cahen P., Sturrock R. F., Ouma J. H., Mwatha J. K., Kimani G., Kariuki H. C., Kazibwe F., Tukahebwa E. and Kabatereine N. B. (2006). Applied and basic research on the epidemiology, morbidity, and immunology of schistosomiasis in fishing communities on Lake Albert, Uganda. Transactions of the Royal Society of Tropical Medicine and Hygiene 100: 216–223
Fitzsimmons C. M., Joseph S., Jones F. M., Reimert C. M., Hoffmann K.␣F., Kazibwe F., Kimani G., Mwatha J. K., Ouma J. H., Tukahebwa E., Kariuki H. C., Vennervald B. J., Kabatereine N. B. and Dunne D. W. (2004). Chemotherapy for schistosomiasis in Ugandan fishermen: treatment can cause a rapid increase in interleukin-5 levels in plasma but decreased levels of eosinophilia and worm-specific immunoglobulin E. Infection and Immunology 72: 4023–4030
Jordan P. (1985). Schistosomiasis: The St. Lucia Project. Cambridge University Press, Cambridge, 442
Kabatereine N. B., Vennervald B. J., Ouma J. H., Kemijumbi J., Butterworth A. E., Dunne D. W. and Fulford A. J. C. (1999). Adult resistance to schistosomiasis mansoni: age-dependence of re-infection remains constant in communities with diverse exposure patterns. Parasitology 118: 101–105
Kabatereine, N. B., 2000. Schistosoma mansoni in a fishing community on the shores of Lake Albert at Butiaba. Uganda: epidemiology, morbidity, re-infection patterns and impact of treatment with praziquantel. PhD Thesis, University of Copenhagen
Kabatereine N. B., Brooker S., Tukahebwa E., Kazibwe F. and Onapa A. (2004). Epidemiology and geography of Schistosoma mansoni in Uganda: implications for planning control. Tropical Medicine and International Health 9: 1–9
Kabatereine N. B., Tukahebwa E., Kazibwe F., Namwangye H., Zaramba S., Brooker S., Stothard J. R., Kamenka C., Whawell S., Webster J. P. and Fenwick A. (2006). Progress towards countrywide control of schistosomiasis and soil-transmitted helminthiasis in Uganda. Transactions of the Royal Society of Tropical Medicine and Hygiene 100: 208–215
Kloos H., Fulford A. J. C., Butterworth A. E., Sturrock R. F., Ouma J. H., Kariuki H. C., Thiongo F. W., Dalton P. R. and Klumpp R. K. (1997). Spatial patterns of Human water contact and Schistosoma mansoni transmission and infection in four rural areas in Machakos district, Kenya. Social Science and Medicine 44: 949–968
Lima Costa M. M. F., Rocha R. S., Coura Filho P. and Katz N. (1993). A 13-year follow-up of treatment and snail control in an area endemic for Schistosoma mansoni in Brasil: incidence of infection and reinfection. Bulletin of the World Health Organization 71: 197–205
Madsen H. (1985). Ecology and Control of African Freshwater Pulmonate Snails; Part 1: Life Cycle and Methodology. Danish Bilharziasis Laboratory, Copenhagen: 49 pp
Madsen H. (1982). Development of egg masses of newly hatched snails of some species of intermediate hosts of schistosomiasis in water conditioned by Helisoma duryi (Wetherby) (Pulmonata: Planorbidae). Malacologia 22: 427–434
Mandahl-Barth G. (1978). Unpublished WHO Document Schisto/WP 78.5. World Health Organization, Geneva
Mandahl-Barth, G., 1958. Intermediate hosts of Schistosoma – African Biomphalaria and Bulinus. World Health Monograph Series, No. 37
Mobarak A. B. (1982). The schistosomiasis problem in Egypt. American Journal of Tropical Medicine and Hygiene 31: 89–91
Ouma, J. H., 1987. Transmission of Schistosoma mansoni in an endemic area of Kenya with special reference to the role of human defaecation behaviour and sanitary practices. PhD Thesis, University of Liverpool
Prentice M. A. (1972). Distribution, prevalence and transmission of schistosomiasis in Uganda. Uganda Medical Journal 1: 136–140
Sleigh A. C., Li X., Jackson S. and Huang K. (1998). Eradication of schistosomiasis in Guangxi, China. 2. Political economy, management strategy and costs, 1953–1992. Bulletin of the World Health Organization 76: 497–508
Stothard J. R., Mgeni A. F., Khamis S., Seto E., Ramsan M. and Rollinson D. (2002). Urinary schistosomiasis in schoolchildren on Zanzibar Island (Unguja), Tanzania: a parasitological survey supplemented with questionnaires. Transactions of the Royal Society of Tropical Medicine and Hygiene 96: 507–514
Stothard J. R., Kabatereine N. B., Tukahebwa E. M., Mathieson W., Webster J. P. and Fenwick A. (2005). Field evaluation of the Meade Readiview handheld microscope for diagnosis of intestinal schistosomiasis in Ugandan school children. American Journal of Tropical Medicine and Hygiene 73: 949–955
Sturrock R. F., Klumpp R. K., Ouma J. H., Butterworth A. E., Fulford A. J. C., Kariuki H. C., Thiongo F. W. and Koech D. (1994). Observations on the effects of different chemotherapy strategies on the transmission of Schistosoma mansoni in Machakos District, Kenya, measured by long-term snail sampling and cercariometry. Parasitology 109: 443–453
Sturrock R. F. (1975). Distribution of the snail Biomphalaria glabrata intermediate host of Schistosoma mansoni within a St. Lucian field habitat. Bulletin of the World Health Organization 52: 267–272
Utzinger J., Bergquist R., Xiao S. H. and Singer B. H. (2003). Sustainable schistosomiasis control – the way forward. Lancet 362: 1932–1934
Webbe G. and El Hak S. (1990). Progress in the control of schistosomiasis in Egypt, 1985–1988. Transactions of the Royal Society of Tropical Medicine and Hygiene 84: 394–400
Webbe, G., 1962. Population studies of intermediate hosts in relation to transmission of bilharzia in East Africa. In “Bilharziasis”, Wolstenholme, G. E. W. & M. O’Connor (eds), A Ciba Foundation Symposium. J & A. Churchill Ltd, London, 7–22
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kazibwe, F., Makanga, B., Rubaire-Akiiki, C. et al. Ecology of Biomphalaria (Gastropoda: Planorbidae) in Lake Albert, Western Uganda: snail distributions, infection with schistosomes and temporal associations with environmental dynamics. Hydrobiologia 568, 433–444 (2006). https://doi.org/10.1007/s10750-006-0224-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-006-0224-y