Abstract
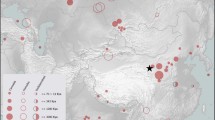
Multiple introductions of a species are thought to enhance its invasion success by increasing genotypic diversity; this involves frequent crossing among different lineages. However, genetic diversity through crossing is less likely in autogamous species. To understand the impact of multiple introductions on the colonization success of autogamous species, we studied hairy bittercress, Cardamine hirsuta, which invaded Japan several decades ago. We detected temporal changes in its population structure using nine microsatellite markers amplified from leaf samples collected from 87 sites between 2009 and 2010, and herbarium specimens collected between 1988 and 2007. To examine whether the phenotypic variation corresponded with the genetic population structure, we also investigated the geographic variation in the lateral stamen number of this species across 49 sites. The present populations can be divided into three genetic groups, which are distributed in northern, eastern, and western Japan. This finding suggests that there are three invasive lineages (North, East, and West) in Japan. The geographic variation in lateral stamen number corresponded to the distributions of these lineages. The former distributions of the North and West lineages mostly corresponded to those found at present, but they were also historically found in eastern Japan. However, the East lineage has apparently expanded into eastern Japan, resulting in a change in dominant lineages over only a few decades. For the autogamous C. hirsuta, multiple introductions contributed toward colonization success over a wider range, which was associated with a local change in the dominant lineages.



Similar content being viewed by others
References
Amsellem L, Noyer JL, Le Bourgeois T, Hossaert-McKey M (2000) Comparison of genetic diversity of the invasive weed Rubus alceifolius poir. (Rosaceae) in its native range and in areas of introduction, using amplified fragment length polymorphism (AFLP) markers. Mol Ecol 9:443–455
Anderson JT, Inouye DW, McKinney AM et al (2012) Phenotypic plasticity and adaptive evolution contribute to advancing flowering phenology in response to climate change. Proc R Soc B Biol Sci 279:3843–3852
Barrett SCH, Colautti RI, Eckert CG (2008) Plant reproductive systems and evolution during biological invasion. Mol Ecol 17:373–383
Bonin A, Bellemain E, Bronken Eidesen P et al (2004) How to track and assess genotyping errors in population genetics studies. Mol Ecol 13:3261–3273
Bossdorf O, Auge H, Lafuma L et al (2005) Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia 144:1–11
Colautti RI, Lau JA (2015) Contemporary evolution during invasion: evidence for differentiation, natural selection, and local adaptation. Mol Ecol 49:1999–2017
Culliney TW (2005) Benefits of classical biological control for managing invasive plants. CRC Crit Rev Plant Sci 24:131–150
Dlugosch KM, Parker IM (2008) Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Mol Ecol 17:431–449
Dormontt EE, Gardner MG, Breed MF et al (2014) Genetic bottlenecks in time and space: reconstructing invasions from contemporary and historical collections. PLoS One 9:e106874
Durka W, Bossdorf O, Prati D, Auge H (2005) Molecular evidence for multiple introductions of garlic mustard (Alliaria petiolata, Brassicaceae) to North America. Mol Ecol 14:1697–1706
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Gao H, Williamson S, Bustamante CD (2007) A Markov chain Monte Carlo approach for joint inference of population structure and inbreeding rates from multilocus genotype data. Genetics 176:1635–1651
Goudet J (1995) FSTAT (Version 1.2): a computer program to calculate F-statistics. J Hered 86:485–486
Goudet J (2001) FSTAT, a program to estimate and test gene diversities and fixation indices (version 2.9.3). http://www.unil.ch/izea/softwares/fstat.html
Grime JP, Hodgson JG, Hunt R (1988) Comparative plant ecology. A functional approach to common British species. Unwin Hyman, London
Grimsby JL, Kesseli R (2010) Genetic composition of invasive Japanese knotweed s.l. in the United States. Biol Invasions 12:1943–1946
Hagenblad J, Hülskötter J, Acharya KP et al (2015) Low genetic diversity despite multiple introductions of the invasive plant species Impatiens glandulifera in Europe. BMC Genet 16:103
Hastings A, Cuddington K, Davies KF et al (2004) The spatial spread of invasions: new developments in theory and evidence. Ecol Lett 8:91–101
Hay AS, Pieper B, Cooke E et al (2014) Cardamine hirsuta: a versatile genetic system for comparative studies. Plant J 78:1–15
Holt RD (2009) Up against the edge: invasive species as testbeds for basic questions about evolution in heterogeneous environments. Mol Ecol 18:4347–4348
Hutchinson WF, van Oosterhout C, Rogers SI, Carvalho GR (2003) Temporal analysis of archived samples indicates marked genetic changes in declining North Sea cod (Gadus morhua). Proc Biol Sci 270:2125–2132
Jaspars-Schrader TW (1982) Het onderscheid tussen Cardamine flexuosa With. en C. hirsuta L. Gorteria 10:213–219
Kolbe JJ, Glor RE, Schettino LRG et al (2004) Genetic variation increases during biological invasion by a Cuban lizard. Nature 431:177–181
Kudoh H (2012) Recent expansion of an invasive plant species, Cardamine hirsuta, in Japan. In: Morita T (ed) Natural history of invasive plants—ecology of invasion and disturbance. Hokkaido University Press, Sapporo, pp 127–148 (in Japanese)
Kudoh H, Ishiguri Y, Kawano S (1992) Cardamine hirsuta L., a new ruderal species introduced into Japan. J Phytogeogr Taxon 40:85–89
Kudoh H, Marhold K, Lihova J (2006) Notes on Cardamine impatiens L., C. flexuosa With., C. hirsuta L. and C. parviflora L. in Japan. Bunrui 6:41–49 (in Japanese with English summary)
Kudoh H, Nakayama M, Lihova J, Marhold K (2007) Does invasion involve alternation of germination requirements? A comparative study between native and introduced strains of an annual Brassicaceae, Cardamine hirsuta. Ecol Res 22:869–875
Lavergne S, Molofsky J (2007) Increased genetic variation and evolutionary potential drive the success of an invasive grass. Proc Natl Acad Sci USA 104:3883–3888
Le Roux JJ, Wieczorek AM, Wright MG, Tran CT (2007) Super-genotype: global monoclonality defies the odds of nature. PLoS One 2:e590
Lelong B, Lavoie C, Jodoin Y, Belzile F (2007) Expansion pathways of the exotic common reed (Phragmites australis): a historical and genetic analysis. Divers Distrib 13:430–437
Lihova J, Marhold K, Kudoh H, Koch MA (2006) Worldwide phylogeny and biogeography of Cardamine flexuosa (Brassicaceae) and its relatives. Am J Bot 93:1206–1221
Lockwood JL, Cassey P, Blackburn T (2005) The role of propagule pressure in explaining species invasions. Trends Ecol Evol 20:223–228
Maki M, Horie S, Yokoyama J (2002) Comparison of genetic diversity between narrowly endemic shrub Menziesia goyozanensis and its widespread congener M. pentandra (Ericaceae). Conserv Genet 3:421–425
Matesanz S, Theiss KE, Holsinger KE, Sultan SE (2014) Genetic diversity and population structure in Polygonum cespitosum: insights to an ongoing plant invasion. PLoS One 9:e93217
Matsuhashi S, Sakai S, Kudoh H (2012) Temperature-dependent fluctuation of stamen number in Cardamine hirsuta (Brassicaceae). Int J Plant Sci 173:391–398
Meimberg H, Milan NF, Karatassiou M et al (2010) Patterns of introduction and adaptation during the invasion of Aegilops triuncialis (Poaceae) into Californian serpentine soils. Mol Ecol 19:5308–5319
Mergeay J, Verschuren D, De Meester L (2006) Invasion of an asexual American water flea clone throughout Africa and rapid displacement of a native sibling species. Proc R Soc B-Biol Sci 273:2839–2844
Moran EV, Alexander JM (2014) Evolutionary responses to global change: lessons from invasive species. Ecol Lett 17:637–649
Nei M, Tajima F, Tateno Y (1983) Accuracy of estimated phylogenetic trees from molecular data 2. Gene frequency data. J Mol Evol 19:153–170
Neuffer B, Hurka H (1999) Colonization history and introduction dynamics of Capsella bursa-pastoris (Brassicaceae) in north america: isozymes and quantitative traits. Mol Ecol 8:1667–1681
Nordborg M, Hu TT, Ishino Y et al (2005) The pattern of polymorphism in Arabidopsis thaliana. PLoS Biol 3:1289–1299
Novak SJ, Mack RN (1993) Genetic variation in Bromus tectorum introduced populations. Heredity 71:167–176
Paetkau D, Calvert W, Stirling I, Strobeck C (1995) Microsatellite analysis of population-structure in Canadian polar bears. Mol Ecol 4:347–354
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Piry S, Alapetite A, Cornuet JM et al (2004) GENECLASS2: a software for genetic assignment and first-generation migrant detection. J Hered 95:536–539
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Pyšek P, Richardson DM (2010) Invasive species, environmental change and management, and health. Annu Rev Environ Resour 35:25–55
R Development Core Team (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN: 3-900051-07-0. http://www.R-project.org/
Roman J, Darling JA (2007) Paradox lost: genetic diversity and the success of aquatic invasions. Trends Ecol Evol 22:454–464
Rosenthal DM, Ramakrishnan AP, Cruzan MB (2008) Evidence for multiple sources of invasion and intraspecific hybridization in Brachypodium sylvaticum (Hudson) Beauv. in North America. Mol Ecol 17:4657–4669
Sakai AK, Allendorf FW, Holt JS et al (2001) The population biology of invasive species. Annu Rev Ecol Syst 32:305–332
Saltonstall K (2002) Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. Proc Natl Acad Sci USA 99:2445–2449
Sato Y, Kudoh H (2013) Relative strength of phenotypic selection on the height and number of flowering-stalks in the rosette annual Cardamine hirsuta (Brassicaceae). J Ecol Environ 36:151–158
Sax DF, Stachowicz JJ, Brown JH et al (2007) Ecological and evolutionary insights from species invasions. Trends Ecol Evol 22:465–471
Sexton JP, McIntyre PJ, Angert AL, Rice KJ (2009) Evolution and ecology of species range limits. Annu Rev Ecol Evol Syst 40:415–436
Simberloff D (2009) The role of propagule pressure in biological invasions. Annu Rev Ecol Evol Syst 40:81–102
Taberlet P, Griffin S, Goossens B et al (1996) Reliable genotyping of samples with very low DNA quantities using PCR. Nucleic Acids Res 24:3189–3194
Verhoeven KJF, Macel M, Wolfe LM, Biere A (2011) Population admixture, biological invasions and the balance between local adaptation and inbreeding depression. Proc R Soc B-Biol Sci 278:2–8
Vitousek PM, Dantonio CM, Loope LL, Westbrooks R (1996) Biological invasions as global environmental change. Am Sci 84:468–478
Wilson JRU, Dormontt EE, Prentis PJ et al (2009) Something in the way you move: dispersal pathways affect invasion success. Trends Ecol Evol 24:136–144
Yatsu Y, Kachi N, Kudoh H (2003) Ecological distribution and phenology of an invasive species, Cardamine hirsuta L. and its native counterpart, Cardamine flexuosa With., in central Japan. Plant Species Biol 18:35–42
Zhang YY, Zhang DY, Barrett SCH (2010) Genetic uniformity characterizes the invasive spread of water hyacinth (Eichhornia crassipes), a clonal aquatic plant. Mol Ecol 19:1774–1786
Acknowledgments
We thank Makino Herbarium and Tohoku University Herbarium for providing specimen samples; A. Hay, S. Horie, T. Yamada, R. Kikuchi, Y. Sakamoto, N. Matsushima, Y. Suyama, A. Matsuo, T. Kawagoe, and M. Katabuchi for help with genetic experiments and analyses; K. Hikosaka for support with the phytotron; and T. Nakashizuka for field support and comments on the manuscript. This study was supported by Grant-in-Aid for The Japan Society for the Promotion of Science Fellows and partly supported by the Sasakawa Scientific Research Grant from The Japan Science Society.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Matsuhashi, S., Kudoh, H., Maki, M. et al. Invasion history of Cardamine hirsuta in Japan inferred from genetic analyses of herbarium specimens and current populations. Biol Invasions 18, 1939–1951 (2016). https://doi.org/10.1007/s10530-016-1139-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-016-1139-9