Abstract
Successfully managing invasive plants in natural systems is extremely difficult. Recently however, progress has been made with an approach focused on changing ecosystem processes through the disturbance regime. We performed a large-scale (3 ha) full-factorial field experiment in densely invaded woodland in Hluhluwe-iMfolozi Park, a savanna reserve in South Africa, to study the effect of fire on the control of the pan-tropical invasive exotic shrub Chromolaena odorata in combination with the conventional method, i.e. manual clearing and herbicide application. We show how fire interacted with the conventional clearing of C. odorata and induced an intense canopy fire that caused a shift from woodland to grassland. After 2.5 years of monitoring, grasses were still dominant and re-invasion minimal. It is important to note that fire without prior clearing did not have the same effect and was not successful in reducing densities of C. odorata. An integrated control practice targeting the species with mechanical and chemical methods, while simultaneously targeting its habitat through fire, effectively controlled dense C. odorata thickets during the course of the experiment. However, this approach transformed regular surface fires into high-intensity canopy fires that are rare in savannas. We discuss how this altered fire regime may threaten native habitats, including fire-sensitive forest patches and riverine woodlands within the savanna mozaic. This is an important dilemma for managers that should not be overlooked and asks for long-term data on the impact of control programs on the native vegetation.
Similar content being viewed by others
Introduction
Invasions of exotic plants form a huge threat to the conservation of biodiversity worldwide (Mack et al. 2000; Rejmánek et al. 2005). Exotic plants not only invade landscapes that are heavily modified by humans, but many protected areas are facing similar threats (Macdonald and Frame 1988; Usher et al. 1988). According to global change scenarios, savannas are among the ecosystems most vulnerable to biotic invasions (Sala et al. 2000). While managing invasions in these species-rich savanna systems is important to biodiversity conservation, control is often time-consuming, labour-intensive, and expensive, while positive results often last for only a short time (Marais et al. 2004; Perrings et al. 2005). We, therefore, urgently need to develop efficient and effective control measures to limit the invasion of exotic plants (Zavaleta et al. 2001). Many control programs aimed at targeting invasive species in the past, gave little consideration of the functioning of the invaded ecosystem. Such programs were often ineffective in controlling the invasive species (Hulme 2006; Buckley 2008). In contrast, approaches that target ecosystem processes, rather than primarily the invasive species, have shown much more promising results (Zavaleta et al. 2001; Paynter and Flanagan 2004; Firn et al. 2008). The core principle of these ecosystem-based approaches is that they manipulate the natural disturbance regimes in the invaded ecosystems, such as flooding, soil disturbance and fire (Buckley et al. 2007; Firn et al. 2008).
Fire is one of the key disturbances in savannas worldwide (Bond and Keeley 2005; Bond et al. 2005). Savannas are characterized by the coexistence of tree and grass communities (Scholes and Archer 1997) and tree densities can vary greatly between savannas types, ranging from closed woodland to open grassland habitat (Scholes and Archer 1997; Sankaran et al. 2005). Bond et al. (2005) showed that fire might be the dominant factor that controls the shift between grassland and closed woodland habitats in savannas. This role of fire is especially strong in mesic savannas with more than 650 mm of rain annually (Sankaran et al. 2005). As a result, fire is widely used as a management tool in savannas to control woody encroachment of grassland habitats by native species (Trollope 1983). In fact, it has been successfully applied to switch woody plant dominated savannas to more open grassland systems (Trollope 1983; Roques et al. 2001). Additionally, fire plays a role in maintaining these open grassland systems because competition with grasses is thought to hamper colonization of woody species (Roques et al. 2001). Therefore, fire could also be an important tool for controlling invasions of exotic woody species in savanna systems.
Most studies on plant invasions and fire in savannas have been performed in Neotropical and Australian savannas, where introduced African pasture grasses alter fuel load and increase fire frequency and intensity thereby disrupting savanna structure and functioning (D’Antonio and Vitousek 1992; Rossiter et al. 2003). In contrast, savanna habitats in Africa and Asia are mostly invaded by Neotropical woody plants, such as Lantana camara (L.) and Chromolaena odorata (L.) King and Robinson (Foxcroft et al. 2009, 2010). These woody invaders differ from the invasions by grasses in their effect on the fire regime. In contrast to the grass invaders in American and Australian systems, they often decrease fuel load and fire frequency by outshading native grasses, but increase the risk of high intensity canopy fires (Brooks et al. 2004). The exotic scrambling shrub Chromolaena odorata, for example, is a species that has been reported to increase vertical continuity of fires in savannas, i.e. lifting grassland fires into tree canopies (Macdonald 1983; Macdonald and Frame 1988). Since C. odorata preferably invades closed woodland and forest margins, it might facilitate grassland fires into these fire-sensitive habitats that do not burn when ecotones are not invaded (Macdonald 1983; Macdonald and Frame 1988). Hence, understanding how woody invaders in African savannas alter the fire regime might not only hold the key to control their invasion, but also increase our insight in the potential threats of this altered fire regime to fire-sensitive habitats.
We performed a large-scale experiment in savanna woodland in Hluhluwe-iMfolozi Park (HiP), a protected reserve in South Africa that was densely invaded by C. odorata. We applied fire in combination with conventional mechanical and chemical control and assessed the effect on densities and re-invasion of C. odorata. Our objectives were two-fold, test if (1) fire can increase the effectiveness of the conventional clearing practice in controlling C. odorata, and (2) test if C. odorata can cause high-intensity canopy fires and how this affects the native vegetation of the invaded habitat.
Methods
Study species
Chromolaena odorata originates from South and Central America and has invaded a wide variety of ecosystems, ranging from tropical rainforests to savannas, in most of the Paleotropics (Kriticos et al. 2005; Raimundo et al. 2007). It is a medium-sized shrub that reaches a height of 1.5–2 m. In its naturalised ranges the species forms dense impenetrable monospecific stands that out-shade most native vegetation (Goodall and Erasmus 1996). In the savanna biome of South Africa the species grows in a variety of vegetation types, including grassland, but it prefers woodland habitat and forest margins and is often found along rivers (Macdonald and Frame 1988; Goodall and Zacharias 2002; Foxcroft et al. 2010). In closed-canopy woodlands and forest margins it becomes a large scrambler that grows into the tree canopy, with light and pithy stems that dry quickly and burn easily (Macdonald and Frame 1988). Even after severe disturbances, such as cutting, the species resprouts rapidly from remaining green stems and/or from the base (Devendra et al. 1998).
Study area
Hluhluwe-iMfolozi Park (HiP) is a 90,000 ha reserve in Kwazulu-Natal, South Africa, falling within the southern African savanna biome (28°00′–28°26′ S and 31°43′–32°09′ E). In HiP, C. odorata is the most dominant and widespread invasive species with, at the time of study, about 20% of the northern section of the park covered with dense monospecific stands (Howison 2009). We selected a study site in one of the more densely invaded areas of this northern section, the Maphumulo area. Average annual rainfall of this area is 800 mm/year and is strongly seasonal with most rain falling between October and March. With this amount of rainfall savannas are highly unstable and the amount of disturbance, especially fire, determines local tree cover (Bond et al. 2005; Sankaran et al. 2005). The northern part of HiP, therefore, represents a mosaic of habitats from open grassland to closed woodland and even forest in absence of fire (Whateley and Porter 1983). In this mosaic C. odorata mostly invades the woodlands and forest margins. We situated our study site in heavily invaded mixed broad-leaved/fine-leaved closed woodland with dominant canopy species including Euclea racemosa, E. divinorum, Combretum molle, Sideroxylon inerme, Acacia robusta, Peltophorum africanum and Berchemia zeyheri. Dominant sub-canopy species were Dicrostachys cinerea, Kraussia floribunda, Rhus pentheri, Gymnosporia senegalensis and Diospyros dicrophylla. The dominant grass species were the tufted grasses Panicum maximum and Eragrostis curvula and the stoloniferous grass Dactylotenium australe.
An important management practice in the reserve is the use of fire to control woody shrub encroachment and remove moribund grass to improve conditions for large grazing herbivores (Conway et al. 2001). In general fire is confined to the grassland and open woodland communities, with closed woodland and forests tending to exclude fire (Balfour and Howison 2001). Controlled burning is generally carried out from June to October, with most fires occurring at the end of the dry season (August/September) and on average 26% of the surface area of the reserve is burned each year (Balfour and Howison 2001). The average fire return period from 1956 to 1996 was 2.9 years for high rainfall areas (~1,000 mm/year) and 3.8 years for lower rainfall areas (~700 mm/year) (Balfour and Howison 2001).
Experimental design
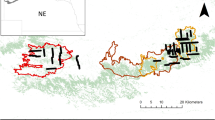
We set up a large-scale (3 ha) experiment with three treatments to control densities of C. odorata (fire (Fi), conventional clearing (CC) and clearing followed by fire (CC + Fi)) and followed the effect of these treatments on densities and re-invasion of C. odorata during subsequent 2.5 years. In addition, we assessed the effect of these treatments on the indigenous vegetation of the invaded habitat. The experiment was set up according to a randomized block design (Fig. 1), with three replicate 1 ha blocks and four 50 × 50 m treatment plots per block, each subjected to one of the three treatments (Fi, CC or CC + Fi) or an experimental control with no management intervention (Control). Blocks were allocated according to differences in initial C. odorata density. Block 1 had the lowest C. odorata density. Block 2 had highest initial densities with almost 100% cover and block 3 was intermediate in initial C. odorata density.
Lay-out of the experiment, showing the position of the blocks and treatments (fire (Fi), conventional clearing followed by fire (CC + Fi), conventional clearing (CC) and the untreated control). The line represents the 10 m wide fire break, the dots indicate the ignition points of the fires and the shaded areas with the arrows indicate the direction of the two fire fronts
We applied the treatments from August to October 2003. The initial clearing was performed in September 2003 according to Working for Water standards and consists of hand-pulling or spraying of seedlings and slashing of established plants followed by herbicide application to the remaining stumps (Van Gils et al. 2004; Euston-Brown et al. 2007). A follow-up clearing following the same procedure as the initial clearing and which is a standard practice in conventional clearing programmes, was performed in May 2005. By accident, the experimental controls were cleared as well during this follow-up. The burning was performed on 13 October 2003 on a dry and hot day, with gentle to moderate wind.
Quantifying fire intensity
We used open calorimeters to measure fire intensities (Wally et al. 2006; Moncrieff et al. 2008) since standard calculations based on heat yield (Byram 1959) could not be used due to lack of data on C. odorata acting as fuel load. Calorimeters existed of a set of aluminium cans with basal area of 60.84 cm2 and 20 ml of water in each can. Each calorimeter was fitted with 3 cans at ground level, at grass canopy height and 1 m above the ground. We placed 4 calorimeters per treatment and measured the volume of water evaporated after burning. Rate of spread was measured by individual observers measuring the time it took for the fire front to pass from one fixed point to the next. Finally, fire intensity was calculated as (rate of spread) × (volume of water evaporated per cm2) × (2.571 kJ, which is the energy required to convert 1 g of water to steam (Weast 1988)). Additionally, flame height was measured with 4 m long poles with pieces of string attached every 20 cm. The highest string burned gave an estimate of flame height.
Monitoring of Chromolaena and native vegetation
We revisited the experiment once every 2 months to monitor Chromolaena and native grass recovery in permanent plots. Thirty permanent sample units of 2 × 5 m were evenly spaced in each of the 50 × 50 m treatment plots. Sample units were monitored 16 times during the course of the experiment, from August 2003 to February 2006. We recorded C. odorata densities as the total number of stems per sample unit under the categories: (1) seedlings (stem diameter < 0.5 cm, stem not yet lignified), (2) young shrubs (stem diameter 0.5–2 cm, stem lignified, <1 m height) and (3) old shrubs (stem diameter > 2 cm, stem lignified, >1 m height).
We assessed the effect of the treatments on native vegetation by monitoring grass recovery, species composition and damage to trees. Together with the Chromolaena records we measured grass height on five 1 m spaced points along a transect in the middle of each of the 30 sample units using a disc pasture meter (Bransby and Tainton 1977). In the fire treatment plots (Fi and CC + Fi) we tagged ninety individual trees of the two most dominant species in our experiment, Dicrostachys cinerea and Euclea racemosa, evenly distributed across the three blocks, and recorded height and stem diameter. One year after our fire treatment, trees were revisited and we assessed percentage top kill and mortality.
Data analysis
We separated the data in four time periods: (1) Pre-treatment (August 2003, n = 1), (2) Months 1–6 after establishment of the treatments (November 2003–March 2004, n = 4), (3) Months 7–18 after establishment of the treatments (April 2004–March 2005, n = 7) and (4) Follow-up clearing, months 19–28 after establishment of the treatments (May 2005–February 2006, n = 4). It is important to note that during the follow-up clearing in May 2005, by accident, the experimental controls were cleared as well. Furthermore in time period 3 grass height was measured during only five of the seven monitoring assessments.
In a separate study we estimated above-ground C. odorata biomass from an allometric regression of above-ground dry weight (g) of C. odorata shrubs with the diameter of the main stem of these shrubs (cm) (y = 77.63*x 2.57, R 2 = 0.95, n = 80, P < 0.001). Based on this regression we estimated total above-ground C. odorata biomass in the treatment plots from the stem count data, using the following average stem diameters for each size class: 0.3 cm for the seedlings, 1.5 cm for the young shrubs and 3 cm for the old shrubs. The grass height data was averaged per sample plot and grass biomass was derived from this using the equation: mean biomass (kg/ha) = 340 + 388.3* mean grass height (cm) (Trollope 1983).
Mixed-effect models were used to test for an overall effect of treatment (Fi, CC, CC-Fi, Control) on total C. odorata biomass for the different time periods separately (pre-treatment, months 1–6, months 7–18, and follow-up). The mixed-effect model allowed us to include random factors (plot, block and date) with hierarchical levels to account for spatial and temporal correlations. We nested plot (n = 4) within block (n = 3) and block within date (n = 16) as a random effect. We used Tukey multiple comparisons to test for contrasts between the levels of the treatments. We performed the same analysis for each of the three size classes (seedlings, young shrubs and old shrubs) separately.
In a second analysis we tested for the effect of fire intensity on densities of C. odorata. We averaged the data for time periods 2 and 3 (months 1–6 and 7–18), excluding pre-treatment and follow-up data and tested per size class (seedlings, young shrubs and old shrubs). To account for the unbalanced design, we averaged the data for blocks 2 and 3 that burned with the high intensity fire and used a one-way ANOVA to compare it with block 1 that burned with the low fire intensity.
In a third analysis we tested for changes in grass biomass over time. We used the same mixed-effect model as we used for the Chromolaena biomass and performed Tukey multiple comparisons to test for contrasts between the levels of the treatments. All analyses were performed in R (Version 2.7.0 (2008-04-22)) (R Development Core Team 2008).
Results
Characteristics of the fire treatments
Two separate fires burned the experiment with different ignition sources and intensities. Block 1 burned with a low intensity and patchy back fire, i.e. a fire that is burning against the prevailing wind direction (Fig. 1). The rate of spread was 0.03 m/s, resulting in a fire intensity of 116 kJ/s/m, which is classified as a very cool fire (Trollope and Potgieter 1985). Flame height was not higher than 20 cm and ash colour was black. The back fire was ignited from the fire break to be able to control the head fire, i.e. a fire burning with the prevailing wind direction that was ignited from the opposite direction (Fig. 1). Unfortunately, the head fire changed direction and missed block 1. When reaching dense stands of cleared C. odorata just outside the experiment this head fire turned from a surface fire to an active canopy fire that burned blocks 2 and 3. Rates of spread were difficult to measure, due to the extreme nature of the fire, and were estimated to be at least 1 m/s. Based on this, fire intensity for blocks 2 and 3 was calculated to be 7,200 kJ/s/m, which is classified as an extremely hot fire (Trollope and Potgieter 1985). However, as in some calorimeters all water evaporated, the fire intensity is presumed to be a minimum estimate. Average flame height in block 2 was 3.6 m and in block 3 average flame height was 1.2 m, ash colour was predominantly grey, white and red in both blocks.
Effect of treatments on C. odorata biomass
The pre-treatment biomass of C. odorata did not differ between treatments (Fig. 2a, F 3,6 = 0.42, P = 0.75). During the first 6 months aboveground biomass of C. odorata was lower in all treatments compared to the control (Fig. 2b, F 3,33 = 20.94, P < 0.001). The clearing treatment and the clearing-followed-by-fire treatment strongly reduced C. odorata biomass in the first 6 months to 26 and 10% of the biomass in the controls, respectively. The fire treatment was much less effective with 60% of biomass present relative to controls. In the following year, months 7–18 (Fig. 2c), biomass in all treatments was still lower than in the controls (F 3,60 = 27.60, P < 0.001). However, C. odorata biomass strongly increased in the clearing treatment and in the fire treatment, reaching 68 and 71% of the biomass of the controls. The clearing-followed-by-fire treatment was still very effective in reducing C. odorata biomass, with only 18% C. odorata biomass present relative to the control. After the follow-up clearing, months 19–28 (Fig. 2d), C. odorata biomass in the clearing-followed-by-fire treatment was further reduced to 1.5% relative to control levels of month 7–18 (F 3,33 = 2.45, P = 0.08). The other treatments were also effectively reduced after the follow-up clearing to 6 and 10% in the fire treatment and in the clearing treatment, respectively, including, by accident, the control itself that was reduced to 13% relative to control levels of month 7–18. The clearing-followed-by-fire treatment remained low during the whole period, while the other treatments started to increase again about one year after the follow-up clearing (data not shown). Figure 3 shows photographs of the clearing-followed-by-fire treatment for each of the time periods: pre-treatment, month 1, month 12 and month 28.
The effect of treatments on total aboveground biomass of C. odorata (g/m2) with time periods analysed separately: pre-treatment (n = 1), months 1–6 (n = 4), months 7–18 (n = 7) and months 19–28 (n = 4) after establishment of the treatments. The last period represents the follow-up clearing. Experimental treatments are shown on the x-axis (fire (Fi), conventional clearing followed by fire (CC + Fi), conventional clearing (CC) and the untreated control). Bars show mean biomass (+SE), letters denote significant differences (P < 0.05)
Effect of treatments per size class
Pre-treatment biomass of C. odorata seedlings (F 3,6 = 0.56, P = 0.66), young shrubs (F 3,6 = 2.04, P = 0.21) and old shrubs (F 3,6 = 0.29, P = 0.83) did not differ between treatments. During the first 6 months after the treatments seedling biomass was low and did not differ between treatments (F 3,33 = 1.39, P = 0.26). In the following year, months 7–18, seedling biomass remained low, except in the clearing treatment (F 3,60 = 14.2, P < 0.001) that showed a sharp increase in seedling recruitment. Biomass of young shrubs was initially reduced as well in all treatments (F 3,33 = 9.49, P < 0.001) to 49, 18 and 41% relative to control levels, in the fire, clearing-followed-by-fire and clearing treatments, respectively. However, in months 7–18 biomasses increased strongly in all treatments to 130, 73 and 107% relative to the control, although the clearing-followed-by-fire treatment was still significantly lower than the other treatments (F 3,60 = 8.06, P < 0.001). Biomass of old shrubs was effectively reduced to 1 and 9% in the clearing-followed-by-fire and clearing treatments, (F 3,33 = 14.1, P < 0.001) during the first 6 months, but was not much reduced in the fire treatment, with 74% of biomass present relative to controls. In the following year, biomass of old shrubs increased in the fire and clearing treatments to 60 and 33% relative to the control. The clearing-followed-by-fire treatment remained low with only 7% biomass still present (F 3,60 = 25.5, P < 0.001). After the follow-up clearing biomasses of seedlings (F 3,33 = 1.54, P = 0.22), young shrubs (F 3,33 = 3.27, P = 0.03) and old shrubs (F 3,33 = 2.23, P = 0.10) were strongly reduced in all treatments, including the controls, with the clearing-followed-by-fire treatment still having the lowest level of C. odorata biomass across all size classes.
Effect of fire intensity on control of C. odorata
Fire intensity influenced the reduction of seedlings and young shrubs, but not of old shrubs. The densities of C. odorata seedlings (Fig. 4a, F 1,118 = 52.7, P < 0.001) and young shrubs (Fig. 4b, F 1,118 = 51.2, P < 0.001) were lower in plots with high fire intensity than with the cool fire. However, the density of old shrubs was not affected by fire intensity (Fig. 4c, F 1,118 < 0.001, P = 0.98).
The effect of fire intensity on aboveground biomass of C. odorata (g/m2) per treatment for each of the size classes: seedlings (a), young shrubs (b) and old shrubs (c). Bars show mean C. odorata biomass (+SE) for low and high fire-intensity. The fire treatment (Fi) is shown in dark bars and the clearing-followed-by-fire treatment (CC + Fi) in light bars. Data is averaged for months 1–18, excluding the pre-treatment and the follow-up data. Note the differences in scale axes
Effects of fire treatments on native vegetation
The high-intensity canopy fire in blocks 2 and 3 of the experiment resulted in a high percentage of top-killed trees (Fig. 5). In these blocks on average 88% of tagged trees were top-killed, including all trees higher then 4 m and tree mortality after 1 year was 27%. In block 1 that burned with the cool fire only 3% of all tagged trees were top-killed, all of which were below 1 m in height. No tree mortalities were observed in this block.
Pre-treatment grass biomass did not differ between treatment plots (Fig. 6a, F 3,6 = 2.29, P = 0.18). During the first 6 months grass biomass was significantly higher in the clearing treatment (Fig. 6b, F 3,33 = 4.16, P = 0.01) and actually increased as compared to pre-treatment levels. In the following year, months 7–18, grass biomass increased in all treatments except in the controls (Fig. 6c, F 3,42 = 4.54, P = 0.008). After the follow-up clearing, months 19–28, grass biomass decreased again in the clearing treatment, while in the fire treatment and in the clearing-followed-by-fire treatment grass biomass remained high (Fig. 6d, F 3,33 = 14.0, P < 0.001).
The effect of treatments on grass biomass (kg/ha) with time periods analysed separately: pre-treatment (n = 1), months 1–6 (n = 4), months 7–18 (n = 5) and months 19–28 (n = 4) after establishment of the treatments. The last period represents the follow-up clearing. Note that 10 kg/ha = 1 g/m2. Experimental treatments are shown on the x-axis (fire (Fi), conventional clearing followed by fire (CC + Fi), conventional clearing (CC) and the untreated control). Bars show mean biomass (+ SE), letters denote significant differences (P < 0.05)
Discussion
The combination of clearing-followed-by-fire (CC + Fi) was very effective in reducing densities of the invasive exotic shrub C. odorata and much more effective than the single treatments (Fi and CC). After 2.5 years of monitoring, minimal re-invasion of C. odorata occurred in the clearing-followed-by-fire treatments in contrast to the other treatments. However, the clearing-followed-by-fire treatment caused a high-intensity canopy fire that severely impacted the native vegetation, causing more than 80% top-kill of native trees and changing the system from a woody-dominated state to a grass-dominated state. These results show that controlling invasive species can come at a high cost of loosing native habitat and managers have to take this into consideration when planning control programs.
The use of fire as a single treatment (Fi) did not control C. odorata. We found that the old C. odorata shrubs were fire-tolerant and able to vigorously re-sprout even after a high-intensity fire (Fig. 4). Densities of seedlings significantly decreased as a result of fire and more so after a high-intensity fire. Mortality by fire, therefore, seems to be more dependent on the age and size of plants than on fire intensity. Moreover, previous research has shown that fire-induced mortality of C. odorata depends on the amount of grass fuel load present. More than 30% grass cover is needed to control sparse to moderate infestations by fire (Goodall and Zacharias 2002). Indeed, high grass cover might induce a higher fire-intensity around the basal stem of the plants, inducing a higher mortality than during a canopy fire, when the aerial parts are most intensely burned.
The use of conventional clearing as single treatment (CC) did reduce C. odorata densities initially, but the species quickly recovered and grew back to biomass levels similar to those in the control plots within the first year. Especially the young shrubs quickly recovered and clearing seemed to be least effective in this size class. Possibly, smaller stems are easily missed when applying herbicide to the cut stump, especially in dense stands, or there is less surface for application of the herbicide, and therefore less penetration to the roots. Also young plants have a higher sprouting ability as was shown in previous work on C. odorata (Kushwaha et al. 1981; Devendra et al. 1998). The follow-up clearing was much more effective in reducing densities of C. odorata than the initial clearing and densities stayed lower for a longer period of time. Therefore, once an area has been cleared, follow-up clearings or controlled burning are essential to prevent re-invasion.
In addition to the control of C. odorata we aimed to increase our understanding of the effects of these control programs on the native vegetation through an altered fire regime. This follows suggestions that the species increases the vertical continuity of fires as a scrambler on native trees (Macdonald 1983; Macdonald and Frame 1988) and has a high tissue flammability due to essential oils that renders the species flammable when moisture-stressed (Goodall and Erasmus 1996; Witkowski and Wilson 2001). In our experiment dense, cleared C. odorata stands, indeed lifted the initial surface fire from block 1 into the tree canopies, thereby transforming the fire into a high-intensity canopy fire that burned blocks 2 and 3 (Fig. 1). It is important to note that C. odorata shrubs in our experiment were cut and left to dry before burning, which is likely to have intensified the resulting canopy fire. The impact on the native vegetation was high as shown in the more than 80% top-kill of the measured native trees. However, native tree mortality in our experiment was relatively low, confirming that many tree species in savanna woodlands are able to regenerate after fire (Scholes and Archer 1997). Hence, although the structure of the habitat was highly affected and changed from woody-dominated to grass-dominated, this effect might not be long-lasting and the woodland may regenerate. However, other invaded vegetation types within the savanna mosaic, such as riverine vegetation and margins of scarp forests that hosts high levels of diversity and endemism (Whateley and Porter 1983; Conway et al. 2001) are poorly adapted to fire. Our results suggest that, whenever C. odorata is cleared in these habitats, either the flammable litter should be removed or the areas must be protected from fire.
An additional effect of the high-intensity fire is that C. odorata seedling densities were significantly reduced (Fig. 4). Interestingly, seedling densities remained low in these treatments for the duration of the experiment, suggesting that seeds in the soil did not survive the high-intensity fire. Previous research has shown that C. odorata seeds do not tolerate exposure to high soil temperatures under experimental conditions (Mbalo and Witkowski 1997). Even though the burned areas might provide suitable germination conditions, it is likely that few viable seeds are left after passage of the fire and new dispersal is required. These findings correspond to earlier reports that fire eliminates the majority of C. odorata seeds in the soil (Epp 1987; Slaats 1995; Witkowski and Wilson 2001). Seedling recruitment was highest in the conventional clearing (CC) treatments, indicating optimal germination conditions below cleared C. odorata litter. Other experimental studies confirm that seedling emergence doubled on soil surfaces mulched with C. odorata twigs, possibly due to higher soil moisture and reduced evaporation on the covered surface (Slaats 1995; Ambika 2002). Therefore, contrary to previous reports (Macdonald 1983) we argue that cleared rather then burned areas provide optimal germination conditions for C. odorata. This is an important result that should be taken into account when planning follow-up clearings so that special care is taken to target seedlings. Alternatively, the clearing-followed-by-fire treatment is an effective means to prevent re-invasion from seeds.
Experimental studies show that competition with grasses hampers re-invasion of C. odorata through a reduction in seed germination and seedling growth (Erasmus and Van Staden 1986; Renrun and Xuejun 1991; te Beest 2010). In the current study a dense sward of native grasses had established in the areas that burned with the highest intensity (Fig. 3). Despite the presence of this grass layer, in the fire treatment (Fi) C. odorata grew back quickly through re-sprouting, while in the integrated treatment (CC + Fi) minimal reinvasion of C. odorata occurred. In the latter treatment re-invasion was solely dependent on seed dispersal and seedling recruitment. This suggests that competition with grasses is highly relevant for preventing C. odorata seedling establishment, and less so for re-sprouting shrubs. In contrast to seedlings, re-sprouting shrubs can use their belowground storage capital and can therefore quickly re-occupy their own gaps (Bond and Midgley 2001). One way for seedlings to establish in habitats with a dense grass layer is to invest in height to avoid light competition. Experimental studies show that C . odorata seedlings indeed strongly invest in stem biomass (Saxena and Ramakrishnan 1984; te Beest et al. 2009). However, they do induce high mortality due to competition for resources (Yadav and Tripathi 1981). Greenhouse and field studies confirmed that only a small fraction of C. odorata seedlings are able to establish in grasslands, but once established, old shrubs can persist within a continuous grass layer for at least several years (te Beest 2010). Interestingly, grasses, notably the shade-tolerant species Panicum maximum, showed a quick recovery in the clearing treatments during the first 6 months (Fig. 6). This might be due to fire exclusion, but also to altered soil communities. Previous research has shown that soil that was pre-cultured by C. odorata stimulated growth of P. maximum (te Beest et al. 2009).
An important message from our experiment is that dense infestations of C. odorata cannot be controlled by applying fire alone. Although this management practice is still widely applied in its invaded range, it likely only enhances the invasion of the species. Conventional clearing can be highly effective in the control of C. odorata if followed by fire. Fire should be applied wisely, however, since it might have undesirable side-effects. We showed that clearing followed by fire had a large impact on the native trees and switched the habitat from woodland to grassland (Fig. 3). Hence, we argue that fire should be excluded from invaded riverine forests and forest margins, especially if cleared, so that C. odorata cannot carry grassland fires into the canopies of these fire-sensitive vegetation types. Moreover, according to a recent study from Zimbabwe the number of exotic invasive species increased with fire frequency because many invasive plants are ruderals that quickly colonize post-burn sites (Masocha et al. 2011). At the same time there might be potential to use C. odorata-induced canopy fires as a tool to convert invaded and encroached grasslands and thickets into a more desirable open grassland state. Lessons can be learned from the integrated control of invasive species and applied to native woody encroachers. Hence, fire as a management tool to control invasive species clearly has desirable and undesirable outcomes that partly depend on characteristics of the fire regime, such as fire intensity and frequency. Long-term monitoring is essential to not only provide necessary data on efficacy of fire control programs but also its impact on native vegetation and potential re-invasion by alien species. Managers have to be aware of these diverse issues when planning control programs.
References
Ambika SR (2002) The influence of environmental factors on seedling growth in Chromolaena odorata. In: Zachariades C, Muniappan R, Strathie LW (eds) Proceedings of the 5th international workshop on biological control and management of Chromolaena odorata. ARC-PPRI, Pretoria, South Africa, pp 100–105
Balfour D, Howison OE (2001) Spatial and temporal variation in a mesic savanna fire regime: responses to variation in annual rainfall. Afr J Range Forage Sci 19:43–51
Bond WJ, Keeley JE (2005) Fire as a global ‘herbivore’: the ecology and evolution of flammable ecosystems. Trends Ecol Evol 20:387–394
Bond WJ, Midgley JJ (2001) Ecology of sprouting in woody plants: the persistence niche. Trends Ecol Evol 16:45–51
Bond WJ, Woodward FI, Midgley GF (2005) The global distribution of ecosystems in a world without fire. New Phytol 165:525–537
Bransby DI, Tainton NM (1977) The disc pasture meter: possible applications in grazing management. Proc Grassland Soc South Afr 12:115–118
Brooks ML, D’Antonio CM, Richardson DM, Grace JB, Keeley JE, DiTomaso JM, Hobbs RJ, Pellant M, Pyke D (2004) Effects of invasive alien plants on fire regimes. Bioscience 54:677
Buckley YM (2008) The role of research for integrated management of invasive species, invaded landscapes and communities. J Appl Ecol 45:397–402
Buckley YM, Bolker BM, Rees M (2007) Disturbance, invasion and re-invasion: managing the weed-shaped hole in disturbed ecosystems. Ecol Lett 10:809–817
Byram GM (1959) Combustion of forest fuels. In: Davis KP (ed) Forest fire: control and use. McGraw-Hill, New York, pp 155–182
Conway A, Balfour D, Dale T, Hartley P, Morrison P, Howison R, Galli N, Wadge M (2001) Hluhluwe-Umfolozi Park management plan. Ezemvelo KZN Wildlife, Pietermaritzburg, South Africa
D’Antonio CM, Vitousek PM (1992) Biological invasions by exotic grasses, the grass fire cycle, and global change. Ann Rev Ecol Syst 23:63–87
Devendra R, Chavan ML, Ramachandra Prasad TV (1998) Growth patterns, sprouting ability and chemical control of Chromolaena odorata. In: Ferrar P, Muniappan R, Jayanth KP (eds) Proceedings of the 4th international workshop on biological control and management of Chromolaena odorata. Agricultural Experiment Station, University of Guam, Mangilao. Guam publication no. 216
Epp GA (1987) The seedbank of Eupatorium odoratum along a successional gradient in a tropical rain forest in Ghana. J Trop Ecol 3:149
Erasmus DJ, Van Staden J (1986) Germination of Chromolaena odorata (L) K and R. Achenes—effect of temperature, imbibition and light. Weed Res 26:75–81
Euston-Brown D, Rathogwa N, Richardson DM (2007) Development of a clearing protocol based on ecological criteria for mesic savannas and sweet grassveld for the working for water programme. South African Dept. of Water Affairs, internal report. http://www.dwa.gov.za/wfw/
Firn J, Rout T, Possingham H, Buckley YM (2008) Managing beyond the invader: manipulating disturbance of natives simplifies control efforts. J Appl Ecol 45:1143–1151
Foxcroft LC, Richardson DM, Rouget M, MacFadyen S (2009) Patterns of alien plant distribution at multiple spatial scales in a large national park: implications for ecology, management and monitoring. Divers Distrib 15:367–378
Foxcroft LC, Richardson DM, Rejmánek M, Pyšek P (2010) Alien plant invasions in tropical and sub-tropical savannas: patterns, processes and prospects. Biol Invasions 12:3913–3933
Goodall JM, Erasmus DJ (1996) Review of the status and integrated control of the invasive alien weed, Chromolaena odorata, in South Africa. Agric Ecosyst Environm 56:151–164
Goodall JM, Zacharias PJK (2002) Managing Chromolaena odorata in subtopical grasslands in KwaZulu-Natal, South Africa. In: Zachariades C, Muniappan R, Strathie LW (eds) Proceedings of the 5th international workshop on biological control and management of Chromolaena odorata. ARC-PPRI, Pretoria, South Africa, pp 120–127
Howison OE (2009) The historical spread and potential distribution of the invasive alien plant Chromolaena odorata in Hluhluwe-iMfolozi park. MSc Thesis, University of Kwazulu-Natal, Durban, South Africa
Hulme PE (2006) Beyond control: wider implications for the management of biological invasions. J Appl Ecol 43:835–847
Kriticos DJ, Yonow T, McFadyen RC (2005) The potential distribution of Chromolaena odorata (Siam weed) in relation to climate. Weed Res 45:246–254
Kushwaha SPS, Ramakrishnan PS, Tripathi RS (1981) Population dynamics of Eupatorium odoratum in successional environments following slash and burn agriculture. J Appl Ecol 18:529–535
Macdonald IAW (1983) Alien trees, shrubs and creepers invading indigenous vegetation in the Hluhluwe-Umfolozi game reserve complex in natal. Bothalia 14:949–959
Macdonald IAW, Frame GW (1988) The invasion of introduced species into nature reserves in tropical savannas and dry woodlands. Biol Conserv 44:67–93
Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710
Marais C, Van Wilgen BW, Stevens D (2004) The clearing of invasive alien plants in South Africa: a preliminary assessment of costs and progress. South Afr J Sci 100:97–103
Masocha M, Skidmore AK, Poshiwa X, Prins HHT (2011) Frequent burning promotes invasions of alien plants into a mesic African savanna. Biol Invasions 13:1641–1648
Mbalo BA, Witkowski ETF (1997) Tolerance to soil temperatures experienced during and after the passage of fire in seeds of Acacia karroo, A. tortilis and Chromolaena odorata: a laboratory study. South Afr J Botany 63:421–425
Moncrieff GR, Kruger LM, Midgley JJ (2008) Stem mortality of Acacia nigrescens induced by the synergistic effects of elephants and fire in Kruger National Park, South Africa. J Trop Ecol 24:655–662
Paynter Q, Flanagan GJ (2004) Integrating herbicide and mechanical control treatments with fire and biological control to manage an invasive wetland shrub, Mimosa pigra. J Appl Ecol 41:615–629
Perrings C, Dalmazzone S, Williamson M (2005) The economics of biological invasions. In: Mooney HA, McNeely JA, Neville L, Schei PJ, Waage J (eds) Invasive alien species: a new synthesis. Island Press, Washington DC, USA, pp 16–35
Raimundo RLG, Fonseca RL, Schachetti-Pereira R, Peterson AT, Lewinsohn TM (2007) Native and exotic distributions of siamweed (Chromolaena odorata) modelled using the genetic algorithm for rule-set production. Weed Sci 55:41–48
R Development Core Team (2008) R: a language and environment for statistical computing (Version 2.7.0 (2008-04-22)). R Foundation for Statistical Computing Vienna, Austria. ISBN 3-900051-07-0. http://www.R-project.org
Rejmánek M, Richardson DM, Higgins SI, Pitcairn M, Grotkopp E (2005) Ecology of invasive plants: state of the art. In: Mooney HA, McNeely JA, Neville L, Schei PJ, Waage J (eds) Invasive alien species: a new synthesis. Island Press, Washington DC, USA, pp 104–162
Renrun W, Xuejun X (1991) Cultural control of Feijicao (Chromolaena odorata (L.) R. M. King and H. Robinson) by planting signalgrass (Brachiaria decumbens Stapf) in Southern Yunnan, People’s Republic of China. In: Muniappan R, Ferrar P (eds) Proceedings of the 2nd international workshop on biological control and management of Chromolaena odorata, Bogor, Indonesia. BIOTROP special publication no. 44
Roques KG, O’Connor TG, Watkinson AR (2001) Dynamics of shrub encroachment in an African savanna: relative influences of fire, herbivory, rainfall and density dependence. J Appl Ecol 38:268–280
Rossiter NA, Setterfield SA, Douglas MM, Hutley LB (2003) Testing the grass-fire cycle: alien grass invasion in the tropical savannas of northern Australia. Divers Distrib 9:169–176
Sala OE, Chapin FS, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R, Lodge DM, Mooney HA, Oesterheld M, Poff NL, Sykes MT, Walker BH, Walker M, Wall DH (2000) Biodiversity—global biodiversity scenarios for the year 2100. Science 287:1770–1774
Sankaran M, Hanan NP, Scholes RJ, Ratnam J, Augustine DJ, Cade BS, Gignoux J, Higgins SI, Le Roux X, Ludwig F, Ardo J, Banyikwa F, Bronn A, Bucini G, Caylor KK, Coughenour MB, Diouf A, Ekaya W, Feral CJ, February EC, Frost PGH, Hiernaux P, Hrabar H, Metzger KL, Prins HHT, Ringrose S, Sea W, Tews J, Worden J, Zambatis N (2005) Determinants of woody cover in African savannas. Nature 438:846–849
Saxena KG, Ramakrishnan PS (1984) Growth and patterns of resource allocation in Eupatorium odoratum L. in the secondary successional environments following slash and burn agriculture (Jhum). Weed Res 24:127–134
Scholes RJ, Archer SR (1997) Tree-grass interactions in savannas. Ann Rev Ecol Syst 28:544
Slaats JJP (1995) Chromolaena odorata fallow in food cropping systems: an agronomic assessment in South-West Ivory Coast. PhD Thesis, Wageningen Agricultural University, The Netherlands. Tropical resource management papers 11
te Beest M (2010) The ideal weed? Understanding the invasion of Chromolaena odorata in a South African savanna. PhD Thesis, University of Groningen, The Netherlands
te Beest M, Stevens N, Olff H, Van der Putten WH (2009) Plant-soil feedback induces shifts in biomass allocation in the invasive plant Chromolaena odorata. J Ecol 97:1281–1290
Trollope WSW (1983) Control of bush encroachment with fire in the arid savannas of south-eastern Africa. PhD thesis, University of Natal, Pietermaritzburg, South Africa
Trollope WSW, Potgieter ALF (1985) Fire behaviour in the Kruger National Park. J Grassland Soc South Afr 2:17–22
Usher MB, Kruger FJ, Macdonald IAW, Loope LL, Brockie RE (1988) The ecology of biological invasions into nature reserves—an introduction. Biol Conserv 44:1–8
Van Gils H, Delfino J, Rugege D, Janssen L (2004) Efficacy of Chromolaena odorata control in a South African conservation forest. South Afr J Sci 100:251–253
Wally AL, Menges ES, Weekley CW (2006) Comparison of three devices for estimating fire temperatures in ecological studies. Appl Veg Sci 9:97–108
Weast RC (1988) Handbook of chemistry and physics: student edition. CRC Press Inc, Boca Raton
Whateley A, Porter RN (1983) The woody vegetation communities of the Hluhluwe-Corridor-Umfolozi game reserve complex. Bothalia 14:745–758
Witkowski ETF, Wilson M (2001) Changes in density, biomass, seed production and soil seed banks of the non-native invasive plant, Chromolaena odorata, along a 15 year chronosequence. Plant Ecol 152:13–27
Yadav AS, Tripathi RS (1981) Population dynamics of the ruderal weed Eupatorium odoratum and its natural regulation. Oikos 36:361
Zavaleta ES, Hobbs RJ, Mooney HA (2001) Viewing invasive species removal in a whole-ecosystem context. Trends Ecol Evol 16:454–459
Acknowledgments
We thank the Ezemvelo KZN Wildlife research and management staff of Hluhluwe-iMfolozi Park for providing support for the study and assisting with the controlled burning. We want to thank the Working for Water Program for performing the initial clearing and the Chromolaena Clearing Project ‘Impi ka Sandanezwe’ for performing the follow-up clearing. Thanks as well to the many people collecting data for this study: K. Mpandza, N. Mbatha, S. Mhlongo, M. Drijfhout, J. van Dorssen, H. Jansma, R. Xaba, T. Shelembe, S. Nkosi, G. Hunther, S. Khumalo, E. Buthelezi, F. Bos, A. Mkwanazi, T. Gumede, A. van Erk, S. Vilakazi, M. Waldram, and the Mellon staff. We thank the ‘Dungbeetle’ research community, the Mammal Research Institute, Białowieża, Poland and the CEES, University of Oslo, Norway for kindly hosting MtB. K. Barton is thanked for assistance with the statistical analyses. This research was funded by the Dutch Scientific Organisation (NWO-Pionier to HO).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
te Beest, M., Cromsigt, J.P.G.M., Ngobese, J. et al. Managing invasions at the cost of native habitat? An experimental test of the impact of fire on the invasion of Chromolaena odorata in a South African savanna. Biol Invasions 14, 607–618 (2012). https://doi.org/10.1007/s10530-011-0102-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-011-0102-z