Abstract
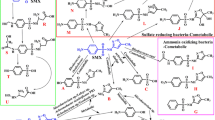
A Nocardioides simplex strain 3E was isolated which totally dechlorinated 2,4,5-trichlorophenoxyacetic acid and was capable of its utilization as the sole source of carbon. The mechanism of 2,4,5-trichlorophenoxyacetic acid degradation by this strain was investigated. Chloroaromatic metabolites that occur in the lag, exponential and stationary growth phases of the strain Nocardioides simplex 3E were isolated and identified bases on a combination of TLC, GC-MS and HPLC data. Decomposition of 2,4,5-trichlorophenoxyacetic acid at the initial stage was shown to proceed by two pathways: via the splitting of the two-carbon fragment to yield 2,4,5-trichlorophenol and the reductive dechlorination to produce 2,4-dichlorophenoxyacetic acid. Hydrolytic dechlorination of 2,4,5-trichlorophenoxyacetic acid was found to yield dichlorohydroxyphenoxyacetic acid, thus pointing to the possible existence of a third branch at the initial stage of degradation of the xenobiotic. 2,4,5-Trichlorophenol and 2,4-dichlorophenoxyacetic acid produced during the metabolism of 2,4,5-trichlorophenoxyacetic acid and in experiments with resting cells are utilized by the strain Nocardioides simplex 3E as growth substrates.
Similar content being viewed by others
Abbreviations
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- 2,4,5-T:
-
2,4,5-trichlorophenoxyacetic acid
- 2,4,5-TCP:
-
2,4,5-trichlorophenol
References
Apajalachi JHA & Salkinoja-Salonen MS (1987) Complete dechlorination of tetra-chlorohydroquinone by cell extracts of pentachlorophenol-induced Rhodococcus chlorophenolicus. J. Bacteriol. 169: 5125–5130
Bradley SG, Brownell GH & Clark J (1973) Genetic homologies among nocardiac and other actinomycetes. Can. J. Microbiol. 19: 1007–1014
Chakrabarty AM (1987) New biotechnological approaches to environmental pollution problems. In: Hollenberg CP & Sahm M (Eds) Microbial Genetic Engineering and Enzyme Technology. Gustaw Fisher, Stuttgart-New York
Collins MD (1985) Analysis of isoprenoid quinones. In: Gottschalk G (Ed) Methods in Microbiology, Vol 18 (pp 329–366)
Collins MD, Dorsch M & Stackebrandt E (1989) Transfer of Pimelobacter tumescens to Terrabacter gen. nov. as Terrabacter tumescens comb. nov. and of Pimelobacter jensenii to Nocardioides as Nocardioides jensenii comb. nov. Int. J. Syst. Bacteriol. 39: 1–6
Collins MD & Stackebrandt E (1989) Molecular taxonomic studies on some LL-diaminopimelic acid-containing coryneforms from herbage: Description of Nocardioides fastidiosa sp. nov. FEMS Microbiol. Letters. 57: 289–294
Farrow JAE & Collins MD (1983) DNA base composition, DNA/DNA homology and long-chain fatty acid studies on Streptococcus thermophilus and Streptococcus salivarius. J. Gen. Microbiol. 129: 1423–1432
Goodfellow M (1971) Numerical taxonomy of some nocardioform bacteria. J. Gen. Microbiol. 69: 33–80
Gordon RE, Barnett DA, Henderhan JE & Pang CH (1974) Nocardia coeliaca, Nocardia autotrophica, and the nocardin strain. Int. J. Syst. Bacteriol. 24: 54–63
Gregersen T (1978) Rapid method for distinction of Gramnegative from Gram-positive bacteria. Eur. J. Appl. Microbiol. Biotechnol. 5: 123–127
Karns JS, Kilbane JJ, Duttagupta S & Chakrabarty AM (1983) Metabolism of halophenols by 2,4,5-trichlorophenoxyacetic acid degrading Pseudomonas cepacia. Appl. Environm. Microbiol. 46: 1176–1181
Kirchner JG (1978) Thin-Layer Chromatography, Vol 1. John Wiley & Sons, Inc., New York
Kroppenstedt RM & Kutzner HJ (1981) Methods for Study of Streptomycetes. Isolation, Characterization, Chemotaxonomy. Brno
Lechevalier MP & Lechevalier HA (1980) The chemotaxonomy of actinomycetes. In: Dietz A & Thayer WD (Eds) Actinomycete Taxonomy. Society for Industrial Microbiology, No 6 (pp 227–291). SUM special publication.
Owen RJ & Pitcher D (1985) Current methods for estimating DNA base composition and levels of DNA-DNA hybridization. In: Goodfellow M & Minnikin DE (Eds) Chemical Methods in Bacterial Systematics (pp 67–93). Academic Press Inc., London
Rochkind ML, Blackburn JM & Sayler GS (1986) Microbial Decomposition of Chlorinated Aromatic Compounds. EPA/600/2–86/090. US Environmental Protection Agency, Cincinnati
Rosenberg A & Alexander M (1980) Microbial metabolism of 2,4,5-trichlorophenoxyacetic acid in soil, soil suspensions, and axemic culture. J. Agr. Food Chem. 28: 297–302
Scheifer KH & Kandler O (1972) Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol. Rew. 36: 402–477
Schlenk H & Hellerman J (1960) Esterification of fatty acids with diazomethane on a small scale. Anal. Chem. 32: 1412–1414
Szokolay A & Madaric A (1969) Eindimensional dunnschicht Chromatographie chlorierter Insektizide auf Fertigplatten mit mehrfacher Entwicklung. J. Chromatogr. 42: 509–519
Suzuki K & Komagata K (1983) Pimelobacter gen. nov.—a new genus of coryne-form bacteria with LL-diaminopimelic acid in the cell wall. J. Gen. Appl. Microbiol. 29: 59–71
Van den Tweel WJJ, Kok JB & de Bont JAM (1987) Reductive dechlorination of 2,4-dichlorobenzoate to 4-chlorobenzoate and hydrolytic dehalogenation of 4-chloro-, 4-bromo-, and 4-iodobenzoate by Alcaligenes denitrificans NTB-1. Appl. Environm. Microbiol. 53: 810–815
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Golovleva, L.A., Pertsova, R.N., Evtushenko, L.I. et al. Degradation of 2,4,5-Trichlorophenoxyacetic acid by a Nocardioides simplex culture. Biodegradation 1, 263–271 (1990). https://doi.org/10.1007/BF00119763
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00119763