Key Points
-
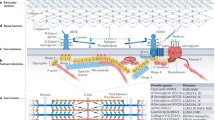
Congenital myasthenic syndromes (CMSs) are a heterogeneous group of disorders that affect the safety margin of neuromuscular transmission — the depolarization that is required for propagation of the change in membrane potential beyond the endplate.
-
Mutations in different synaptic molecules give rise to CMS. These molecules include choline acetyltransferase (CHAT), acetylcholinesterase (ACHE), the different acetylcholine receptor (ACHR) subunits and the AChR-associated molecule RAPSYN.
-
In CMSs that are caused by CHAT mutations, the amplitudes of the endplate potential declined abnormally with increases of firing frequency and showed a slow recovery. These properties pointed to a defect in the resynthesis or vesicular packaging of ACh, an idea that led to the subsequent discovery of mutations in CHAT being responsible for the abnormal phenotype.
-
In CMSs that are caused by ACHE mutations, the reduced degradation of acetylcholine causes the appearance of repetitive and decrementing muscle action potential in response to nerve stimulation. These properties of the action potential stem from repeated rebinding of ACh to the AChR. Importantly, mutations in COLQ, the gene that encodes a collagen tail that anchors ACHE to the basal lamina, also cause a similar CMS.
-
CMSs that are caused by AChR mutations fall in three broad categories. Two of them — slow-channel and fast-channel CMSs — are characterized by kinetic alterations in the receptor, which affect the safety margin for transmission. The third type of mutation does not show significant kinetic alterations; their effect might be mediated by structural alterations or effects on the level of channel expression.
-
CMSs that are related to rapsyn mutations are not accompanied by kinetic alterations on the AChR. Their effect might be related to the abnormal postsynaptic clustering of receptors.
-
Besides shedding light on the pathogenic mechanisms behind CMS, the candidate-gene approach has also provided unexpected insights into the function of different synaptic proteins. In addition, the genetic study of CMS is a paramount example of the power of this approach to bridge clinical, physiological, genetic and morphological analyses to unravel basic mechanisms of human disease.
Abstract
The analysis of congenital myasthenic syndromes (CMSs) has disclosed a diverse array of molecular targets at the motor endplate and has delineated their contribution to synaptic function. Clinical, electrophysiological and morphological studies have paved the way for detecting CMS-related mutations in proteins such as choline acetyltransferase, acetylcholinesterase, the acetylcholine receptor and rapsyn, and studies of the mutant proteins have allowed us to correlate the effects of the mutations with predicted alterations in protein structure. Here, we review the symptomatology of CMSs, consider the factors that impair neuromuscular transmission, survey the mutations that have been uncovered in the different synaptic proteins, and consider the functional implications of the identified mutations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Croxen, R., Vincent, A., Newsom-Davis, J. & Beeson, D. Myasthenia gravis in a woman with congenital AChR deficiency due to ε-subunit mutation. Neurology 58, 1563–1565 (2002).
Salpeter, M. M. in The Vertebrate Neuromuscular Junction (ed. Salpeter, M. M.) 1–54 (Alan Liss, New York, 1987).
Flucher, B. E. & Daniels, M. P. Distribution of Na+ channels and ankyrin in neuromuscular junctions is complementary to that of acetylcholine receptors and the 43 kd protein. Neuron 3, 163–175 (1989).
Ruff, R. L. Sodium channel slow inactivation and the distribution of sodium channels on skeletal muscle fibres enable the performance properties of different skeletal muscle fibre types. Acta Physiol. Scand. 156, 159–168 (1996).
Martin, A. R. Amplification of neuromuscular transmission by postjunctional folds. Proc. R. Soc. Lond. B 258, 321–326 (1994).
Wood, S. J. & Slater, C. P. Safety factor at the neuromuscular junction. Prog. Neurobiol. 64, 393–429 (2001).
Engel, A. G., Ohno, K. & Sine, S. M. in Myasthenia Gravis and Myasthenic Disorders (ed. Engel, A. G.) 251–297 (Oxford Univ. Press, New York, 1999).
Katz, B. Nerve, Muscle, and Synapse (McGraw-Hill, New York, 1966).
Engel, A. G. The investigation of congenital myasthenic syndromes. Ann. NY Acad. Sci. 681, 425–434 (1993).
Greer, M. & Schotland, M. Myasthenia gravis in the newborn. Pediatrics 26, 101–108 (1960).
Byring, R. F. et al. Congenital myasthenic syndrome associated with episodic apnea and sudden infant death. Neuromuscul. Disord. 12, 548–553 (2002).
Mora, M., Lambert, E. H. & Engel, A. G. Synaptic vesicle abnormality in familial infantile myasthenia. Neurology 37, 206–214 (1987).
Okuda, T. et al. Identification and characterization of the high-affinity choline transporter. Nature Neurosci. 3, 120–125 (2000).
Apparsundaram, S., Ferguson, S. M., George, A. L. Jr & Blakely, R. D. Molecular cloning of a human, hemicholinium-3-sensitive choline transporter. Biochem. Biophys. Res. Commun. 276, 862–867 (2000).
Oda, Y., Nakanishi, I. & Deguchi, T. A complementary DNA for human choline acetyltransferase induces two forms of enzyme with different molecular weights in cultured cells. Brain Res. Mol. Brain Res. 16, 287–294 (1992).
Erickson, J. D. et al. Functional identification of a vesicular acetylcholine transporter and its expression from a 'cholinergic' gene locus. J. Biol. Chem. 269, 21929–21932 (1994).
Reimer, R. J., Fon, A. E. & Edwards, R. H. Vesicular neurotransmitter transport and the presynaptic regulation of quantal size. Curr. Opin. Neurobiol. 8, 405–412 (1998).
Ohno, K. et al. Choline acetyltransferase mutations cause myasthenic syndrome associated with episodic apnea in humans. Proc. Natl Acad. Sci. USA 98, 2017–2022 (2001). This is the first description of mutations in CHAT in humans. This study also identifies the kinetic abnormalities in expressed CHAT mutants that impair its catalytic efficiency and impair ACh resynthesis.
Schmidt, C. et al. Congenital myasthenic syndrome due to a novel missense mutation in the gene encoding choline acetyltransferase. Neuromuscul. Disord. 13, 245–251 (2003).
Maselli, R. A. et al. Choline acetyltransferase mutations in myasthenic syndrome due to deficient acetycholine resynthesis. Muscle Nerve 27, 180–187 (2003).
Eiden, L. E. The cholinergic gene locus. J. Neurochem. 70, 2227–2240 (1998).
Massoulié, J., Pezzementi, L., Bon, S., Krejci, E. & Valette, F. -M. Molecular and cellular biology of cholinesterases. Prog. Neurobiol. 41, 31–91 (1993).
Massoulie, J. et al. The polymorphism of acetylcholinesterase: post-translational processing, quaternary associations and localization. Chem. Biol. Interact. 119-120, 29–42 (1999).
Bon, S., Coussen, F. & Massoulié, J. Quaternary associations of acetylcholinesterase. II. The polyproline attachment domain of the collagen tail. J. Biol. Chem. 272, 3016–3021 (1997).
Krejci, E. et al. The mammalian gene of acetylcholinesterase-associated collagen. J. Biol. Chem. 272, 22840–22847 (1997).
Krejci, E. et al. Primary structure of a collagenic tail peptide of Torpedo acetylcholinesterase: co-expression with catalytic subunit induces the production of collagen-tailed forms in transfected cells. EMBO J. 10, 1285–1293 (1991).
Ohno, K., Brengman, J. M., Tsujino, A. & Engel, A. G. Human endplate acetylcholinesterase deficiency caused by mutations in the collagen-like tail subunit (ColQ) of the asymmetric enzyme. Proc. Natl Acad. Sci. USA 95, 9654–9659 (1998). This is the first report of the cloning of COLQ cDNA, chromosomal localization and determination of the genomic structure of COLQ , and identification of truncating mutations in COLQ that result in ACHE deficiency.
Prockop, D. J. & Kivirikko, K. I. Collagens: molecular biology, diseases, and potentials for therapy. Annu. Rev. Biochem. 64, 403–434 (1995).
Deprez, P. N. & Inestrosa, N. C. Two heparin-binding domains are present on the collagenic tail of asymmetric acetylcholinesterase. J. Biol. Chem. 270, 11043–11046 (1995).
Ohno, K., Engel, A. G., Brengman, J. M., et al. The spectrum of mutations causing endplate acetylcholinesterase deficiency. Ann. Neurol. 47, 162–170 (2000).
Kimbell, L. M., Ohno, K., Rotundo, R. L. & Engel, A. G. Transplanting mutant human collagenic tailed acetylcholinesterase onto the frog neuromuscular junction: evidence for an attachment defect in a congenital myasthenic syndrome. Mol. Biol. Cell 12, Suppl. 161a (2001).
Arikawa-Hirasawa, E., Rossi, S. G., Rotundo, R. L. & Yamada, Y. Absence of acetylcholinesterase at the neuromuscular junctions of perlecan-null mice. Nature Neurosci. 5, 119–123 (2002).
Legay, C. et al. Collagen-tailed forms of acetylcholinesterase are anchored to the basal lamina by a dual mechanism involving perlecan and MuSK. Mol. Biol. Cell Abstr. 13, 394a–395a (2002).
Engel, A. G. et al. A newly recognized congenital myasthenic syndrome attributed to a prolonged open time of the acetylcholine-induced ion channel. Ann. Neurol. 11, 553–569 (1982).
Katz, B. & Miledi, R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J. Physiol. (Lond.) 231, 549–574 (1973). An early classical study explaining why inhibition of ACHE prolongs the synaptic response.
Hutchinson, D. O. et al. Congenital endplate acetylcholinesterase deficiency. Brain 116, 633–653 (1993).
Katz, B. & Thesleff, S. A study of the 'desensitization' produced by acetylcholine at the motor end-plate. J. Physiol. (Lond.) 138, 63–80 (1957). The first quantitative description of the desensitization phenomenon in which response to agonist declines in the continued presence of agonist. This paper also demonstrated positive cooperativity in the instantaneous response to agonist.
Maselli, R. A. & Soliven, B. C. Analysis of the organophosphate-induced electromyographic response to repetitive nerve stimulation: paradoxical response to edrophonium and D-tubocurarine. Muscle Nerve 14, 1182–1188 (1991).
Salpeter, M. M., Kasprzak, H., Feng, H. & Fertuck, H. End-plates after esterase inactivation in vivo: correlation between esterase concentration, functional response and fine structure. J. Neurocytol. 8, 95–115 (1979).
Donger, C. et al. Mutation in the human acetylcholinesterase-associated gene, COLQ, is responsible for congenital myasthenic syndrome with end-plate acetylcholinesterase deficiency. Am. J. Hum. Genet. 63, 967–975 (1998). Also cloned COLQ cDNA, identified the chromosomal locus of COLQ and observed a missense mutation in COLQ that caused partial endplate ACHE deficiency.
Ohno, K., Brengman, J. M., Felice, K. J., Cornblath, D. R. & Engel, A. G. Congenital endplate acetylcholinesterase deficiency caused by a nonsense mutation and an A-to-G splice site mutation at position +3 of the collagen-like tail subunit gene (COLQ): how does G at position +3 result in aberrant splicing? Am. J. Hum. Genet. 65, 635–644 (1999).
Shapira, Y. A. et al. The novel COLQ mutations and variation of phenotypic expressivity due to G240X. Neurology 58, 603–609 (2002).
Ishigaki, K. et al. Two novel mutations in the ColQ gene causing endplate acetylcholinesterase deficiency. Neuromuscul. Disord. Abstr. 11, 666–667 (2001).
Brengman, J. M. et al. Neomutations in the collagenic tail subunit (ColQ) of acetylcholinesterase. Neurology 56, Suppl. 3, A60–A61 (2001).
Anderson, C. R. & Stevens, C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J. Physiol. (Lond.) 235, 655–691 (1973).
Ohno, K. et al. Congenital myasthenic syndrome caused by prolonged acetylcholine receptor channel openings due to a mutation in the M2 domain of the ε subunit. Proc. Natl Acad. Sci. USA. 92, 758–762 (1995). The first mutation identified to cause a CMS. A slow-channel syndrome was shown to result from a Thr to Pro mutation in TMD2 of the ε-subunit. Individual channel opening episodes were markedly prolonged, and openings in the absence of agonist increased in frequency. The findings explained the presence of endplate myopathy and the profound weakness of the patient.
Engel, A. G. et al. New mutations in acetylcholine receptor subunit genes reveal heterogeneity in the slow-channel congenital myasthenic syndrome. Hum. Mol. Genet. 5, 1217–1227 (1996).
Sine, S. M. et al. Mutation of the acetylcholine receptor α-subunit causes a slow-channel myasthenic syndrome by enhancing agonist binding affinity. Neuron 15, 229–239 (1995).
Wang, H. -L. et al. Mutation in the M1 domain of the acetylcholine receptor α-subunit decreases the rate of agonist dissociation. J. Gen. Physiol. 109, 757–766 (1997).
Milone, M. et al. Slow-channel syndrome caused by enhanced activation, desensitization, and agonist binding affinity due to mutation in the M2 domain of the acetylcholine receptor α-subunit. J. Neurosci. 17, 5651–5665 (1997).
Gomez, C. M. et al. A β-subunit mutation in the acetylcholine receptor gate causes severe slow-channel syndrome. Ann. Neurol. 39, 712–723 (1996).
Croxen, R. et al. Mutations in different functional domains of the human muscle acetylcholine receptor α-subunit in patients with the slow-channel congenital myasthenic syndrome. Hum. Mol. Genet. 6, 767–774 (1997).
Ohno, K. et al. Slow-channel congenital myasthenic syndrome caused by a novel mutation in the acetylcholine receptor ε-subunit. Neurology Abstr. 50, A432 (1998).
Ohno, K. et al. Slow-channel mutations in the center of the M1 transmembrane domain of the acetylcholine receptor α-subunit. Neurology Abstr. 54, Suppl. 3, A183 (2000).
Gomez, C. M. et al. Novel δ- and β-subunit acetylcholine receptor mutations in the slow-channel syndrome demonstrate phenotypic variability. Soc. Neurosci. Abstr. 24, 484 (1998).
Gomez, C. M. et al. Novel δ-subunit mutation in slow-channel syndrome causes severe weakness by novel mechanism. Ann. Neurol. 51, 102–112 (2002).
Jackson, M. B. Perfection of a synaptic receptor: kinetics and energetics of the acetylcholine receptor. Proc. Natl Acad. Sci. USA. 86, 2199–2203 (1989). This paper rationalized the need for two ACh binding sites per receptor using the concept that the open state binds ACh more tightly than the closed state. It also further explained that at least one of the binding sites has to release ACh quickly to rapidly terminate the response.
Sine, S. M., Claudio, T. & Sigworth, F. J. Activation of Torpedo acetylcholine receptors expressed in mouse fibroblasts: single-channel current kinetics reveal distinct agonist binding affinities. J. Gen. Physiol. 96, 395–437 (1990). Provides the complete analysis of AChR kinetics by fitting a kinetic scheme to single-channel open and closed dwell times. The deduced rate constants showed distinct agonist binding affinities for the two binding sites, and disclosed rapid rates of ACh binding and channel opening.
Sieb, J. P., Milone, M. & Engel, A. G. Effects of the quinoline derivatives quinine, quinidine, and chloroquine on neuromuscular transmission. Brain. Res. 712, 179–189 (1996).
Fukudome, T., Ohno, K., Brengman, J. M. & Engel, A. G. Quinidine normalizes the open duration of slow-channel mutants of the acetylcholine receptor. Neuroreport 9, 1907–1911 (1998). Proof that quinidine, acting as a long-lived open-channel blocker of AChR, normalizes the burst open duration of slow-channel mutants at clinically attainable levels. This study provided the rationale for treating slow-channel CMS with quinidine.
Harper, C. M. & Engel, A. G. Quinidine sulfate therapy for the slow-channel congenital myasthenic syndrome. Ann. Neurol. 43, 480–484 (1998).
Harper, C. M., Fukudome, T. & Engel, A. G. Treatment of slow channel congenital myasthenic syndrome with fluoxetine. Neurology (in the press).
Brejc, K. et al. Crystal structure of ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 411, 269–276 (2001). The first atomic structural determination of a pentameric acetylcholine binding domain. The soluble protein, released from glia of a freshwater snail, is homologous to the ligand-binding domain in the superfamily of pentameric ligand-gated channels. The structure confirms that the ACh binding site is formed at subunit interfaces — with residue side chains contributing from both faces — and provides atomic coordinates of residues that contribute to agonist and antagonist binding.
Sine, S. M. The nicotinic receptor ligand binding domain. J. Neurobiol. 53, 431–446 (2002).
Uchitel, O. et al. Congenital myasthenic syndromes. II. A syndrome attributed to abnormal interaction of acetylcholine with its receptor. Muscle Nerve 16, 1293–1301 (1993).
Jones, S. F. & Kwanbunbumpen, S. Some effects of nerve stimulation and hemicholinium on quantal transmitter release at the mammalian neuromuscular junction. J. Physiol. (Lond.) 207, 51–61 (1970).
Ohno, K. et al. Congenital myasthenic syndrome caused by decreased agonist binding affinity due to a mutation in the acetylcholine receptor ε subunit. Neuron 17, 157–170 (1996). The first identified mutation underlying fast-channel CMS. Single-channel kinetic analysis has shown that a mutation of a conserved Pro in the binding-site region diminished agonist affinity for the open state with little change in affinity of the closed state. Efficiency and rate of channel opening were shown to be profoundly impaired despite the robust expression of the mutant gene.
Shen, X. -M. et al. Mutation causing severe myasthenia reveals functional asymmetry of AChR signature Cys-loops in agonist binding and gating. J. Clin. Invest. 111, 497–505 (2003). This study showed the functional coupling of the α- and δ-subunit Cys-loops — positioned near or within the TMDs — to the remote extracellular ligand binding site. A V132L mutation in the α-Cys-loop profoundly affects closed-state affinity, whereas mutation of the equivalent Val in the δ-subunit impairs channel gating.
Wang, H. -L. et al. Acetylcholine receptor M3 domain: stereochemical and volume contributions to channel gating. Nature Neurosci. 2, 226–233 (1999).
Milone, M. et al. Mode switching kinetics produced by a naturally occurring mutation in the cytoplasmic loop of the human acetylcholine receptor ε-subunit. Neuron 20, 575–588 (1998).
Wang, H. -L. et al. Fundamental gating mechanism of nicotinic receptor channel revealed by mutation causing a congenital myasthenic syndrome. J. Gen. Physiol. 116, 449–460 (2000). Using a new method for kinetic analysis of single-channel currents this study obtained kinetic parameters for individual epochs of receptor activity. It identified a region of the AChR exposed to the cytoplasm that produces uniformity of AChR activation episodes, and highlighted the concept of a corrugated energy landscape governing the gating mechanism.
Brownlow, S. et al. Acetylcholine receptor δ subunit mutations underlie a fast-channel myasthenic syndrome and arthrogryposis multiplex congenita. J. Clin. Invest. 108, 125–130 (2001).
Sine, S. M. et al. Naturally occurring mutations at the acetylcholine receptor binding site independently alter ACh binding and channel gating. J. Gen. Physiol. 120, 483–496 (2002).
Zhong, W., Gallivan, J. P., Zhang, Y., Li, L., Lester, H. A. & Dougherty, D. A. From ab initio quantum mechanics to molecular neurobiology: a cation-π binding site in the nicotinic receptor. Proc. Natl Acad. Sci. USA 95, 12088–12093 (1998).
Sine, S. M., Wang, H. -L. & Bren, N. Lysine scanning mutagenesis delineates structure of nicotinic receptor binding domain. J. Biol. Chem. 277, 29210–29223 (2002). Scanning Lys mutagenesis was used to determine residue disposition in the hydrophobic core, or on the protein surface, in the ligand-binding domain of the human AChR. The resulting side-chain register was used in combination with homology modelling based on the ACHBP structure. Established equivalence between residues in β-sheet structures in the receptor and ACHBP, and deduced an atomic structural model of the human AChR.
Thomsen, R. H. & Wilson, D. F. Effect of 4-aminopyridine and 3,4-diaminopyridineon transmitter release at the neuromuscular junction. J. Pharmacol. Exp. Therap. 227, 260–265 (1983).
Engel, A. G., Ohno, K., Bouzat, C., Sine, S. M. & Griggs, R. G. End-plate acetylcholine receptor deficiency due to nonsense mutations in the ε subunit. Ann. Neurol. 40, 810–817 (1996). Provides evidence that the persistent expression of the foetal AChR γ-subunit can partially compensate for null mutations in both alleles of the AChR ε-subunit and can therefore rescue the phenotype.
Ohno, K. et al. Congenital myasthenic syndromes due to heteroallelic nonsense/missense mutations in the acetylcholine receptor ε subunit gene: identification and functional characterization of six new mutations. Hum. Mol. Genet. 6, 753–766 (1997).
Witzemann, V. et al. Acetylcholine receptor ε subunit deletion causes muscle weakness and atrophy in juvenile and adult mice. Proc. Natl Acad. Sci. USA. 93, 13286–13291 (1996).
Croxen, R. et al. Endplate γ- and ε-subunit mRNA levels in AChR deficiency syndrome due to ε-subunit null mutations. Brain 124, 1362–1372 (2001).
Ohno, K. et al. Myasthenic syndromes in Turkish kinships due to mutations in the acetylcholine receptor. Ann. Neurol. 44, 234–241 (1998).
Abicht, A. et al. A common mutation (ε1267delG) in congenital myasthenic patients of Gipsy ethnic origin. Neurology 53, 1564–1569 (1999).
Middleton, L. et al. Congenital myasthenic syndromes linked to chromosome 17p are caused by defects in acetylcholine receptor ε subunit gene. Neurology 53, 1076–1082 (1999).
Croxen, R. et al. Novel functional ε-subunit polypeptide generated by a single nucleotide deletion in acetylcholine receptor deficiency congenital myasthenic syndrome. Ann. Neurol. 46, 639–647 (1999).
Shen, X. -M. et al. Congenital myasthenic syndrome caused by low-expressor fast-channel AChR δ subunit mutation. Neurology 59, 1881–1888 (2002).
Ealing, J. et al. Mutations in congenital myasthenic syndromes reveal an ε subunit C-terminal cysteine, C470, crucial for maturation and surface expressions of adult AChR. Hum. Mol. Genet. 11, 3087–3096 (2002). This study shows that any mutation that truncates the ε-subunit upstream of the carboxy-terminal Cys 470 residue inhibits AChR expression.
Abicht, A., Stucka, R., Schmidt, C., et al. A newly identified chromosomal microdeletion and an N-box mutation of the AChRε gene cause a congenital myasthenic syndrome. Brain 125, 1005–1013 (2002).
Ohno, K., Anlar, B. & Engel, A. G. Congenital myasthenic syndrome caused by a mutation in the Ets-binding site of the promoter region of the acetylcholine receptor ε subunit gene. Neuromuscul. Disord. 9, 131–135 (1999).
Nichols, P. R. et al. Mutation of the acetylcholine receptor ε-subunit promoter in congenital myasthenic syndrome. Ann. Neurol. 45, 439–443 (1999). This report, and reference 84, provide the best available evidence that the neuregulin signalling pathway regulates synaptic transcription.
Duclert, A., Savatier, N., Schaeffer, L. & Changeux, J. -P. Identification of an element crucial for the sub-synaptic expression of the acetylcholine receptor ε-subunit gene. J. Biol. Chem. 271, 17433–17438 (1996).
Schaeffer, L., Duclert, N., Huchet-Dymanus, M. & Changeux, J. -P. Implication of a multisubunit Ets-related transcription factor in synaptic expression of the nicotinic acetylcholine receptor. EMBO J. 17, 3078–3090 (1998).
Fromm, L. & Burden, S. J. Synapse-specific and neuregulin-induced transcription require an Ets site that binds GABPα/GAPBβ. Genes Dev. 12, 3074–3083 (1998).
Quiram, P. et al. Mutation causing congenital myasthenia reveals acetylcholine receptor β/δ subunit interaction essential for assembly. J. Clin. Invest. 104, 1403–1410 (1999).
Ohno, K. & Engel, A. G. Congenital myasthenic syndromes: gene mutations. Neuromuscul. Disord. 12, 807–811 (2002).
Froehner, S. C., Luetje, C. W., Scotland, P. B. & Patrick, J. The postsynaptic 43K protein clusters muscle nicotinic acetylcholine receptors in Xenopus oocytes. Neuron 5, 403–410 (1990).
Gautam, M. et al. Failure of postsynaptic specialization to develop at neuromuscular junctions of rapsyn-deficient mice. Nature 377, 232–236 (1995).
Willman, R. & Fuhrer, C. Neuromuscular synaptogenesis. Cell. Mol. Life Sci. 59, 1296–1316 (2002).
Marchand, S., Bignami, F., Stetzkowski-Marden, F. & Cartaud, J. The myristoylated protein rapsyn is cotargeted with the nicotinic acetylcholine receptor to the postsynaptic membrane via the exocytic pathway. J. Neurosci. 20, 521–528 (2000).
Marchand, S., Devillers-Thiéry, A., Pons, S., Changeux, J. -P. & Cartaud, J. Rapsyn escorts the nicotinic acetylcholine receptor along the exocytic pathway via association with lipid rafts. J. Neurosci. 22, 8891–8901 (2002).
Maimone, M. M. & Merlie, J. P. Interaction of the 43 kd postsynaptic protein with all subunits of the muscle nicotinic acetylcholine receptor. Neuron 11, 53–66 (1993).
Maimone, M. M. & Enigk, R. E. The intracellular domain of the nicotinic acetylcholine receptor α subunit mediates its coclustering with rapsyn. Mol. Cell. Neurosci. 14, 340–354 (1999).
Cartaud, A., Coutant, S., Petrucci, T. C. & Cartaud, J. Evidence for in situ and in vitro association between β-dystroglycan and the subsynaptic 43K rapsyn protein. Consequence for acetylcholine receptor clustering at the synapse. J. Biol. Chem. 273, 11321–11326 (1998).
Ramarao, M. K. & Cohen, J. B. Mechanism of nicotinic acetylcholine receptor cluster formation by rapsyn. Proc. Natl Acad. Sci. USA 95, 4007–4012 (1998).
Ramarao, M. K., Bianchetta, M. J., Lanken, J. & Cohen, J. B. Role of rapsyn tetratrichopeptide repeat and coiled-coil domains in self-association and nicotinic acetylcholine receptor clustering. J. Biol. Chem. 276, 7475–7483 (2001).
Bartoli, M. & Cohen, J. B. Identification of the modular domains of rapsyn binding to nicotinic acetylcholine receptor (AChR) and to dystroglycan. Soc. Neurosci. Abstr. 27, 904.16 (2001).
Bartoli, M., Ramarao, M. K. & Cohen, J. B. Interactions of the rapsyn RING-H2 domain with dystroglycan. J. Biol. Chem. 276, 24911–24917 (2001).
Lee, Y. I., Swope, S. L. & Ferns, M. J. Rapsyn's C-terminal domain mediates MuSK–induced phosphorylation of the AChR. Mol. Biol. Cell Abstr. 13, 395a (2002).
Yun, K. & Wold, B. Skeletal muscle determination and differentiation: story of a core regulatory network and its content. Curr. Opin. Cell Biol. 8, 877–889 (1996).
Ohno, K. et al. Rapsyn mutations in humans cause endplate acetylcholine receptor deficiency and myasthenic syndrome. Am. J. Hum. Genet. 70, 875–885 (2002). First report of RAPSYN mutations in humans.
Ohno, K., Sadeh, M., Blatt, I., Brengman, J. M. & Engel, A. G. E-box mutations in RAPSN promoter region in eight cases with congenital myasthenic syndrome. Hum. Mol. Genet. 12, 739–748 (2003). First report of E-box mutations in humans.
Gautam, M. et al. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell 85, 525–535 (1996).
Burgess, R. W., Nguyen, Q. T., Son, Y. J., Lichtman, J. W. & Sanes, J. R. Alternatively spliced isoforms of nerve- and muscle-derived agrin: their roles at the neuromuscular junction. Neuron 23, 33–44 (1999).
Glass, D. J. et al. Agrin acts via MuSK receptor complex. Cell 85, 513–523 (1996).
Apel, E. D., Glass, D. J., Moscosco, L. M., Yancopoulos, G. D. & Sanes, J. R. Rapsyn is required for MuSK signaling and recruits synaptic components to a MuSK-containing scaffold. Neuron 18, 623–625 (1997).
Smith, C. L., Mittaud, P., Prescott, E. D., Fuhrer, C. & Burden, S. J. Src, Fyn, and Yes are not required for neuromuscular synapse formation but are necessary for stabilization of agrin-induced clusters of acetylcholine receptors. J. Neurosci. 21, 3151–3160 (2001).
Sandrock, A. W. et al. Maintenance of acetylcholine receptor number by neuregulins at the neuromuscular junction in vivo. Science 276, 599–603 (1997).
Si, J., Luo, Z. & Mei, L. Induction of acetylcholine receptor gene expression by ARIA requires activation of mitogen-activated protein kinase. J. Biol. Chem. 271, 19752–19759 (1996).
Altiok, N., Altiok, K. & Changeux, J. -P. Heregulin-stimulated acetylcholine receptor gene expression in muscle: requirement for MAP kinase and evidence for parallel inhibitory pathway independent electrical activity. EMBO J. 16, 717–725 (1997).
Belluardo, N. et al. Neuromuscular junction disassembly and muscle fatigue in mice lacking neurotrophin-4. Mol. Cell. Neurosci. 18, 56–67 (2001).
Gonzales, M. et al. Disruption of Trkb-mediated signaling induces disassembly of potsynaptic receptor clusters at neuromuscular junctions. Neuron 24, 567–583 (1999).
Newey, S. A. et al. A novel mechanism for modulating synaptic gene expression: differential localization of α-dystrobrevin transcripts in skeletal muscle. Mol. Cell. Neurosci. 17, 127–140 (2001).
Grady, R. M., Merlie, J. P. & Sanes, J. R. Subtle neuromuscular defects in utrophin-deficient mice. J. Cell Biol. 136, 871–882 (1997).
Adams, M. E. et al. Absence of α-syntrophin leads to structurally aberrant neuromuscular synapses deficient in utrophin. J. Cell Biol. 150, 1385–1398 (2000).
Croxen, R. et al. Recessive inheritance and variable penetrance of slow-channel congenital myasthenic syndromes. Neurology 59, 162–168 (2002).
Karlin, A. Emerging structure of the nicotinic acetylcholine receptors. Nature Rev. Neurosci. 3, 102–114 (2002).
Unwin, N., Miyazawa, A., Li, J. & Fujiyoshi, Y. Activation of the nicotinic acetylcholine receptor involves a switch in conformation of the α subunits. J. Mol. Biol. 319, 1165–1176 (2002).
Acknowledgements
Work carried out in the authors' laboratories was supported by grants to A.G.E. and S.M.S. from the National Institutes of Health, and a Muscular Dystrophy Association grant to A.G.E.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
LocusLink
high-affinity choline transporter
OMIM
Swiss-Prot
FURTHER INFORMATION
Encyclopedia of Life Sciences
Glossary
- COMPOUND MUSCLE ACTION POTENTIAL
-
A group of simultaneous action potentials from several muscle fibres in the same area.
- QUANTAL RELEASE
-
A term commonly used to state the fact that neurotransmitters are released in discrete packets or quanta. Each of these quanta corresponds to the fusion of a single synaptic vesicle with the presynaptic membrane so as to release its content.
- MINIATURE ENDPLATE POTENTIAL
-
The change in potential that a single quantum of transmitter elicits on the muscle membrane.
- READILY RELEASABLE QUANTA
-
Synaptic vesicles that, on arrival of a nerve impulse, are available for rapid fusion with the presynaptic membrane. They are docked to the membrane and have been biochemically primed for release.
- BULBAR
-
Related to the medulla oblongata.
- INTERICTAL
-
A term that refers to events that occur between attacks or paroxysms.
- QUANTAL CONTENT
-
The number of quanta that are released per action potential.
- DECAY TIME CONSTANT
-
The initial decayn of an excitatory postsynaptic potential can usually be fit by a single exponential function that describes how quickly the potential decays.
- BASAL LAMINA
-
A component of the neuromuscular junction that surrounds the muscle fibre. It is mainly produced by the muscle cells and consists of collagen, proteoglycans and other molecules of the extracellular matrix.
- REFRACTORY PERIOD
-
The time immediately after the action potential during which the membrane repolarizes through inactivation of voltage-gated sodium channels and through activation of voltage-gated potassium channels.
- OPHTHALMOPARESIS
-
Paralysis of the muscles that control eye movements.
- CYS-LOOP
-
A 15-residue domain, the boundary of which are two cysteine residues that form a disulphide bond. This loop is a signature of the receptor superfamily that includes ionotropic acetylcholine, GABA (γ-aminobutyric acid), glycine and serotonin receptors.
- MISSENSE MUTATIONS
-
Mutations that result in the substitution of an amino acid in a protein.
- TETRATRICO PEPTIDE REPEATS
-
Degenerate motifs of 34 amino acids that are thought to form helix–turn structures, each with a 'knob' and a 'hole', therefore acting as helix-associating domains and mediating protein–protein interactions.
- RING-H2 DOMAIN
-
One of a class of protein domains that consist of two loops that are held together at their base by Cys and His residues that complex two zinc ions.
- CIS-ACTING
-
Regulatory genetic element that is located in the same DNA molecule as the gene that is being regulated.
- FRAMESHIFT MUTATION
-
The addition or deletion of a nucleotide, which shifts the reading frame during translation such that the protein sequence from that point onwards is altered.
- HAPLOTYPE
-
The allelic configuration of multiple genetic markers that are present on a single chromosome of a given subject.
- MICROSATELLITE
-
A class of repetitive DNA that is made up of repeats that are 2–8 nucleotides in length. They are frequently used as molecular markers in studies of population genetics.
- FOUNDER EFFECT
-
Genetic variability observed in a population founded by a small, non-representative sample of a larger population.
- LUCIFERASE REPORTER ASSAY
-
An assay used to indirectly measure the expression of a gene of interest. The firefly luciferase gene is placed downstream of the relevant promoter. As the catalytic action of luciferase emits light, the levels of the enzyme can be quantified by measuring the light intensity.
Rights and permissions
About this article
Cite this article
Engel, A., Ohno, K. & Sine, S. Sleuthing molecular targets for neurological diseases at the neuromuscular junction. Nat Rev Neurosci 4, 339–352 (2003). https://doi.org/10.1038/nrn1101
Issue Date:
DOI: https://doi.org/10.1038/nrn1101
This article is cited by
-
COLQ-related congenital myasthenic syndrome: An integrative view
neurogenetics (2023)
-
Congenital Myasthenic Syndromes in 2018
Current Neurology and Neuroscience Reports (2018)
-
SRSF1 and hnRNP H antagonistically regulate splicing of COLQ exon 16 in a congenital myasthenic syndrome
Scientific Reports (2015)