Abstract
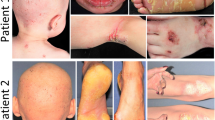
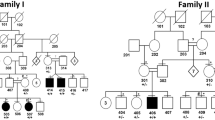
Erythrokeratodermia variabilis (EKV, OMIM 133200) is an autosomal dominant genodermatosis with considerable intra- and interfamilial variability1. It has a disfiguring phenotype characterized by the independent occurrence of two morphologic features: transient figurate red patches and localized or generalized hyperkeratosis (Fig. 1). Both features can be triggered by external factors such as trauma to the skin. After initial linkage to the RH locus on 1p (Refs 2,3 ), EKV was mapped to an interval of 2.6 cM on 1p34-p35, and a candidate gene ( GJA4 ) encoding the gap junction protein α-4 (connexin 31, Cx31) was excluded by sequence analysis4. Evidence in mouse suggesting that the EKV region harbours a cluster of epidermally expressed connexin genes5,6 led us to characterize the human homologues of GJB3 (encoding Cx31) and GJB5 (encoding Cx31.1). GJB3, GJB5 and GJA4 were localized to a 1.1-Mb YAC in the candidate interval. We detected heterozygous missense mutations in GJB3 in four EKV families leading to substitution of a conserved glycine by charged residues (G12R and G12D), or change of a cysteine (C86S). These mutations are predicted to interfere with normal Cx31 structure and function, possibly due to a dominant inhibitory effect. Our results implicate Cx31 in the pathogenesis of EKV, and provide evidence that intercellular communication mediated by Cx31 is crucial for epidermal differentiation and response to external factors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mendes da Costa, S. Erythro- et keratodermia variabilis in a mother and a daughter. Acta Derm. Venereol. 6, 255–261 (1925).
van der Schroeff, J.G. et al. Genetic linkage between erythrokeratodermia variabilis and Rh locus. Hum. Genet. 68, 165– 168 (1984).
van der Schroeff, J.G., van Leeuwen-Cornelisse, I., van Haeringen, A. & Went, L.N. Further evidence for localization of the gene of erythrokeratodermia variabilis. Hum. Genet. 80, 97–98 (1988).
Richard, G. et al. Linkage studies in erythrokeratodermias: fine mapping, genetic heterogeneity and analysis of candidate genes. J. Invest. Dermatol . 109, 666–671 ( 1997).
Haefliger, J.A. et al. Four novel members of the connexin family of gap junction proteins. Molecular cloning, expression, and chromosome mapping. J. Biol. Chem. 267, 2057–2064 (1992).
Hennemann, H. et al. Two gap junction genes, connexin 31.1 and 30.3, are closely linked on mouse chromosome 4 and preferentially expressed in skin. J. Biol. Chem. 267, 17225–17233 (1992).
Bruzzone, R., White, T.W. & Paul, D.L. Connections with connexins: the molecular basis of direct intercellular signaling. Eur. J. Biochem. 238, 1–27 (1996).
Goodenough, D.A., Goliger, J.A. & Paul, D.L. Connexins, connexons, and intercellular communication. Annu. Rev. Biochem. 65, 475– 502 (1996).
Goliger, J.A. & Paul, D.L. Expression of gap junction proteins cx26, cx31.1, cx37, and cx43 in developing and mature rat epidermis. Dev. Dyn. 200, 1–13 ( 1994).
Butterweck, A., Elfgang, K., Willeke, K. & Traub, O. Differential expression of the gap junction proteins connexin45, -43, -40, -31, and -26 in mouse skin. Eur. J. Cell. Biol. 65, 152– 163 (1994).
Risek, B., Klier, F.G. & Gilula, N.B. Multiple gap junction are utilized during rat skin and hair development. Development 116, 639 –651 (1992).
Brissette, J.L., Kumar, N.M., Gilula, N.B., Hall, J.E. & Dotto, G.P. Switch in gap junction protein expression is associated with selective changes in junctional permeability during keratinocyte differentiation. Proc. Natl Acad. Sci. USA 91, 6453–6457 (1994).
Guo, H., Acevedo, P., Parsa, F.D. & Bertram, J.S. Gap-junctional protein connexin 43 is expressed in dermis and epidermis of human skin: differential modulation by retinoids. J. Invest. Dermatol. 99, 460–467 (1992).
Masgrau-Peya, E., Salomon, D., Saurat, J.H. & Meda, P. In vivo modulation of connexins 43 and 26 of human epidermis by topical retinoic acid treatment. J. Histochem. Cytochem. 45 , 1207–1215 (1997).
Larson, D.M., Wrobleski, M.J., Sagar, G.D., Westphale, E.M. & Beyer, E.C. Differential regulation of connexin 43 and connexin 37 in endothelial cells by cell density, growth, and TGF-β1. Am. J. Physiol. 272, C405– 415 (1997).
Reed, K.E. et al. Molecular cloning and functional expression of human connexin 37, an endothelial cell gap junction protein. J. Clin. Invest. 91, 997–1004 ( 1993).
Hoh, J.H., John, S.A. & Revel, J.P. Molecular cloning and characterization of a new member of the gap junction gene family, connexin-31. J. Biol. Chem. 266, 6524–6531 (1991).
Hennemann, H., Schwarz, H.J. & Willecke, K. Characterization of gap junction genes expressed in F9 embryonic carcinoma cells: molecular cloning of mouse connexin31 and -45 cDNAs. Eur. J. Cell Biol. 57, 51– 58 (1992).
Yeager, M. & Nicholson, B.J. Structure of gap junction intercellular channels. Curr. Opin. Struct. Biol. 6, 183 –192 (1996).
Bergoffen, J. et al. Connexin mutations in X-linked Charcot-Marie-Tooth disease. Science 262, 2039–2042 (1993).
Kelsell, D.P. et al. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature 387, 80– 83 (1997).
Shiels, A., Mackay, D., Ionides, A., Berry, V. & Moore, A. A missense mutation in the human connexin 50 gene (GJA8) underlies autosomal dominant "zonular pulverulent" cataract, on chromosome 1q. Am. J. Hum. Genet. 62, 526– 532 (1998).
Verselis, V.K., Ginter, C.S. & Bargiello, T.A. Opposite voltage gating polarities of two closely related connexins. Nature 368, 348– 351 (1994).
Denoyelle, F. et al. Prelingual deafness: high prevalence of a 30delG mutation in the connexin 26 gene. Hum. Mol. Genet. 6, 2173–2177 (1997).
Zelante, L. et al. Connexin 26 mutations associated with the most common form of non-syndromic neurosensory autosomal recessive deafness (DFNB1) in Mediterraneans. Hum. Mol. Genet. 6, 1605– 1609 (1997).
Deschênes, M. et al. Altered trafficking of mutant connexin 32. J. Neuroscience 17, 9077–9084 ( 1997).
Suchyna, T.M., Xu, L.X., Gao, F., Fourtner, C.R. & Nicholson, B.J. Identification of a proline residue as a transduction element involved in voltage gating of gap junctions. Nature 365, 847–849 (1993).
Krawczak M. & Cooper D.N. The human gene mutation database. Trends Genet. 13, 121– 122 (1997).
Richards, B. et al. Multiplex PCR amplification from the CFTR gene using DNA prepared from buccal brushes/swabs. Hum. Mol. Genet. 2, 159–163 (1993).
Itin, P., Levy, C.A., Sommacal-Schopf, D. & Schnyder, U.W. Family study of erythrokeratodermia figurata variabilis. Hautarzt 43, 500–504 ( 1992).
Acknowledgements
The authors are grateful to the families for their generous participation in our studies, and to the Foundation for Ichthyosis and Related Skin Types and the National Registry for Ichthyosis for patient referral. We also appreciate the expert research nursing assistance by M. Miller and M. Anderson, and outstanding technical services of G. Poy in oligonucleotide synthesis and DNA sequencing. D.H. and P.I. were supported by the Swiss National Science Foundation.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Richard, G., Smith, L., Bailey, R. et al. Mutations in the human connexin gene GJB3 cause erythrokeratodermia variabilis. Nat Genet 20, 366–369 (1998). https://doi.org/10.1038/3840
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/3840
This article is cited by
-
Ichthyosis
Nature Reviews Disease Primers (2023)
-
Identification of a CDH12 potential candidate genetic variant for an autosomal dominant form of transgrediens and progrediens palmoplantar keratoderma in a Tunisian family
Journal of Human Genetics (2020)
-
Aneuploidy-inducing gene knockdowns overlap with cancer mutations and identify Orp3 as a B-cell lymphoma suppressor
Oncogene (2020)
-
Molecular cloning and characterization of amh and dax1 genes and their expression during sex inversion in rice-field eel Monopterus albus
Scientific Reports (2015)
-
Erythrokeratodermia Variabilis et Progressiva Allelic to Oculo-Dento-Digital Dysplasia
Journal of Investigative Dermatology (2015)