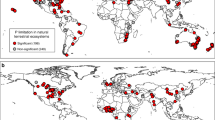
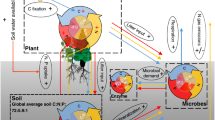
Abstract
In most terrestrial ecosystems, plant growth is limited by nitrogen and phosphorus. Adding either nutrient to soil usually affects primary production, but their effects can be positive or negative. Here we provide a general stoichiometric framework for interpreting these contrasting effects. First, we identify nitrogen and phosphorus limitations on plants and soil microorganisms using their respective nitrogen to phosphorus critical ratios. Second, we use these ratios to show how soil microorganisms mediate the response of primary production to limiting and non-limiting nutrient addition along a wide gradient of soil nutrient availability. Using a meta-analysis of 51 factorial nitrogen–phosphorus fertilization experiments conducted across multiple ecosystems, we demonstrate that the response of primary production to nitrogen and phosphorus additions is accurately predicted by our stoichiometric framework. The only pattern that could not be predicted by our original framework suggests that nitrogen has not only a structural function in growing organisms, but also a key role in promoting plant and microbial nutrient acquisition. We conclude that this stoichiometric framework offers the most parsimonious way to interpret contrasting and, until now, unresolved responses of primary production to nutrient addition in terrestrial ecosystems.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
LeBauer, D. S. & Treseder, K. K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89, 371–379 (2008).
Harpole, W. S. et al. Nutrient co-limitation of primary producer communities. Ecol. Lett. 14, 852–862 (2011).
Elser, J. J. et al. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 10, 1135–1142 (2007).
Gorban, A. N., Pokidysheva, L. I., Smirnova, E. V. & Tyukina, T. A. Law of the minimum paradoxes. Bull. Math. Biol. 73, 2013–2044 (2010).
Danger, M., Daufresne, T., Lucas, F., Pissard, S. & Lacroix, G. Does Liebig’s law of the minimum scale up from species to communities? Oikos 117, 1741–1751 (2008).
Marleau, J. N., Guichard, F. & Loreau, M. Emergence of nutrient co-limitation through movement in stoichiometric meta-ecosystems. Ecol. Lett. 18, 1163–1173 (2015).
Farrior, C. E. et al. Resource limitation in a competitive context determines complex plant responses to experimental resource additions. Ecology 94, 2505–2517 (2013).
Schmidt, I. K., Michelsen, A. & Jonasson, S. Effects on plant production after addition of labile carbon to arctic/alpine soils. Oecologia 112, 305–313 (1997).
Schmidt, I. K., Michelsen, A. & Jonasson, S. Effects of labile soil carbon on nutrient partitioning between an arctic graminoid and microbes. Oecologia 112, 557–565 (1997).
Kuzyakov, Y. & Xu, X. L. Competition between roots and microorganisms for nitrogen: mechanisms and ecological relevance. New Phytol. 198, 656–669 (2013).
Wild, B. et al. Amino acid production exceeds plant nitrogen demand in Siberian tundra. Environ. Res. Lett. 13, 034002 (2018).
Manzoni, S., Trofymow, J. A., Jackson, R. B. & Porporato, A. Stoichiometric controls on carbon, nitrogen, and phosphorus dynamics in decomposing litter. Ecol. Monogr. 80, 89–106 (2010).
Spohn, M. & Kuzyakov, Y. Phosphorus mineralization can be driven by microbial need for carbon. Soil. Biol. Biochem. 61, 69–75 (2013).
Sakala, W. D., Cadisch, G. & Giller, K. E. Interactions between residues of maize and pigeonpea and mineral N fertilizers during decomposition and N-mineralization. Soil. Biol. Biochem. 32, 679–688 (2000).
Chen, Y. et al. Nitrogen mineralization as a result of phosphorus supplementation in long-term phosphate deficient soil. Appl. Soil Ecol. 106, 24–32 (2016).
Brundrett, M. C. Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320, 37–77 (2009).
Smith, S. E. & Read, D. J. Mycorrhizal Symbiosis 3rd ed (Academic Press, Cambridge, MA, 2010).
Franklin, O. et al. Forests trapped in nitrogen limitation—an ecological market perspective on ectomycorrhizal symbiosis. New Phytol. 203, 657–666 (2014).
Ågren, G. I., Wetterstedt, J. Å. & Billberger, M. F. K. Nutrient limitation on terrestrial plant-modeling the interaction between nitrogen and phosphorus. New Phytol. 194, 953–960 (2012).
Čapek, P., Kotas, P., Manzoni, S. & Šantrůčková, H. Drivers of phosphorus limitation across soil microbial communities. Funct. Ecol. 30, 1705–1713 (2016).
Gusewell, S. N. P. ratios in terrestrial plants: variation and functional significance. New Phytol. 164, 243–266 (2004).
Elser, J. J., Fagan, W. F., Kerkhoff, A. J., Swenson, N. G. & Enquist, B. J. Biological stoichiometry of plant production: metabolism, scaling and ecological response to global change. New Phytol. 186, 593–608 (2010).
Ågren, G. I. The C:N/P stoichiometry of autotrophs—theory and observations. Ecol. Lett. 7, 185–191 (2004).
Klausmeier, C. A., Litchman, E., Daufresne, T. & Levin, S. A. Optimal nitrogen-to-phosphorus stoichiometry of phytoplankton. Nature 429, 171–174 (2004).
Minden, V. & Kleyer, M. Internal and external regulation of plant organ stoichiometry. Plant Biol. 16, 897–907 (2014).
Cotner, J. B., Makino, W. & Biddanda, B. A. Temperature affects stoichiometry and biochemical composition of Escherichia coli. Microb. Ecol. 52, 26–33 (2006).
Cherif, M. & Loreau, M. When microbes and consumers determine the limiting nutrient of autotrophs: a theoretical analysis. Proc. Biol. Sci. 276, 487–497 (2009).
Yan, Z. B. et al. Effects of nitrogen and phosphorus supply on growth rate, leaf stoichiometry, and nutrient resorption of Arabidopsis thaliana. Plant Soil 388, 147–155 (2015).
Makino, W., Cotner, J. B., Sterner, R. W. & Elser, J. J. Are bacteria more like plants or animals? Growth rate and resource dependence of bacterial C: N: P stoichiometry. Funct. Ecol. 17, 121–130 (2003).
Yan, J. et al. The mechanism for exclusion of Pinus massoniana during the succession in subtropical forest ecosystems: light competition or stoichiometric homoeostasis? Sci. Rep. 5, 10994 (2015).
Hall, E. K. et al. Linking microbial and ecosystem ecology using ecological stoichiometry: a synthesis of conceptual and empirical approaches. Ecosystems 14, 261–273 (2010).
Zechmeister-Boltenstern, S. et al. The application of ecological stoichiometry to plant–microbial–soil organic matter transformations. Ecol. Monogr. 85, 133155 (2015).
Güsewell, S., Gessner, M. O., Gusewell, S. & Gessner, M. O. N. P. ratios influence litter decomposition and colonization by fungi and bacteria in microcosms. Funct. Ecol. 23, 211–219 (2009).
Demoling, F., Figueroa, D. & Baath, E. Comparison of factors limiting bacterial growth in different soils. Soil. Biol. Biochem. 39, 2485–2495 (2007).
Manzoni, S. et al. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol. 196, 79–91 (2012).
Sinsabaugh, R. L., Manzoni, S., Moorhead, D. L., Richter, A. & Elser, J. Carbon use efficiency of microbial communities: stoichiometry, methodology and modelling. Ecol. Lett. 16, 930–939 (2013).
Doi, H. et al. Integrating elements and energy through the metabolic dependencies of gross growth efficiency and the threshold elemental ratio. Oikos 119, 752–765 (2010).
Isaac, M. E., Hinsinger, P. & Harmand, J. M. Nitrogen and phosphorus economy of a legume tree-cereal intercropping system under controlled conditions. Sci. Total Environ. 434, 71–78 (2012).
Augusto, L., Delerue, F., Gallet-Budynek, A. & Achat, D. L. Global assessment of limitation to symbiotic nitrogen fixation by phosphorus availability in terrestrial ecosystems using a meta-analysis approach. Global. Biogeochem. Cycles 27, 804–815 (2013).
Diáková, K. et al. Variation in N2 fixation in subarctic tundra in relation to landscape position and nitrogen pools and fluxes. Arctic Antarct. Alp. Res. 48, 111–125 (2016).
Šantrůčková, H., Rejmánková, E., Pivničková, B. & Snyder, J. M. Nutrient enrichment in tropical wetlands: shifts from autotrophic to heterotrophic nitrogen fixation. Biogeochemistry 101, 295–310 (2010).
Lagrange, A., L’Huillier, L. & Amir, H. Mycorrhizal status of Cyperaceae from New Caledonian ultramafic soils: effects of phosphorus availability on arbuscular mycorrhizal colonization of Costularia comosa under field conditions. Mycorrhiza 23, 655–661 (2013).
Koide, R. T., Huenneke, L. F., Hamburg, S. P. & Mooney, H. A. Effects of applications of fungicide, phosphorus and nitrogen on the structure and productivity of an annual serpentine plant community. Funct. Ecol. 2, 335 (1988).
Mooshammer, M. et al. Adjustment of microbial nitrogen use efficiency to carbon: nitrogen imbalances regulates soil nitrogen cycling. Nat. Commun. 5, 3694 (2014).
Saggar, S., Parfitt, R. L., Salt, G. & Skinner, M. F. Carbon and phosphorus transformations during decomposition of pine forest floor with different phosphorus status. Biol. Fertil. Soils 27, 197–204 (1998).
Dietrich, K., Spohn, M., Villamagua, M. & Oelmann, Y. Nutrient addition affects net and gross mineralization of phosphorus in the organic layer of a tropical montane forest. Biogeochemistry 136, 223–236 (2017).
Nave, L. E., Vance, E. D., Swanston, C. W. & Curtis, P. S. Impacts of elevated N inputs on north temperate forest soil C storage, C/N, and net N-mineralization. Geoderma 153, 231–240 (2009).
Hatch, D. J., Lovell, R. D., Antil, R. S., Jarvis, S. C. & Owen, P. M. Nitrogen mineralization and microbial activity in permanent pastures amended with nitrogen fertilizer or dung. Biol. Fertil. Soils 30, 288–293 (2000).
Johnson, D. W., Edwards, N. T. & Todd, D. E. Nitrogen mineralization, immobilization, and nitrification following urea fertilization of a forest soil under field and laboratory conditions. Soil Sci. Soc. Am. J. 44, 610 (1980).
Adams, M. A. & Attiwill, P. M. Patterns of nitrogen mineralization in 23-year old pine forest following nitrogen fertilizing. For. Ecol. Manage. 7, 241–248 (1984).
Marklein, A. R. & Houlton, B. Z. Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol. 193, 696–704 (2012).
Feller, I. C., McKee, K. L., Whigham, D. F. & O’Neill, J. P. Nitrogen vs. phosphorus limitation across an ecotonal gradient in a mangrove forest. Biogeochemistry 62, 145–175 (2003).
Keuskamp, J. A., Feller, I. C., Laanbroek, H. J., Verhoeven, J. T. A. & Hefting, M. M. Short- and long-term effects of nutrient enrichment on microbial exoenzyme activity in mangrove peat. Soil. Biol. Biochem. 81, 38–47 (2015).
Watanabe, T., Urayama, M., Shinano, T., Okada, R. & Osaki, M. Application of ionomics to plant and soil in fields under long-term fertilizer trials. + 4, 781 (2015).
Chang, Y. et al. Nutrients resorption and stoichiometry characteristics of different-aged plantations of Larix kaempferi in the Qinling Mountains, central China. PLoS ONE 12, e0189424 (2017).
Kulaev, I., Vagabov, V. & Kulakovskaya, T. New aspects of inorganic polyphosphate metabolism and function. J. Biosci. Bioeng. 88, 111–129 (1999).
Xu, X. et al. Convergence of microbial assimilations of soil carbon, nitrogen, phosphorus, and sulfur in terrestrial ecosystems. Sci. Rep. 5, 17445 (2015).
Manzoni, S. et al. Optimal metabolic regulation along resource stoichiometry gradients. Ecol. Lett. 20, 1182–1191 (2017).
Olde Venterink, H. Productivity increase upon supply of multiple nutrients in fertilization experiments; co-limitation or chemical facilitation? Plant Soil 408, 515–518 (2016).
Bracken, M. E. S. et al. Signatures of nutrient limitation and co-limitation: responses of autotroph internal nutrient concentrations to nitrogen and phosphorus additions. Oikos 124, 113–121 (2015).
Dutta, P. S., Kooi, B. W. & Feudel, U. Multiple resource limitation: nonequilibrium coexistence of species in a competition model using a synthesizing unit. Theor. Ecol. 7, 407–421 (2014).
Xu, X. F., Thornton, P. E. & Post, W. M. A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems. Glob. Ecol. Biogeogr. 22, 737–749 (2013).
Wassen, M., Van Der Vliet, R. & Verhoeven, J. Nutrient limitation in the Biebrza fens and floodplain (Poland). ACTA Bot. Neerl. 47, 241–253 (1998).
Wright, S. J. et al. Potassium, phosphorus, or nitrogen limit root allocation, tree growth, or litter production in a lowland tropical forest. Ecology 92, 161–1625 (2011).
Campo, J. & Vázquez-Yanes, C. Effects of nutrient limitation on above-ground carbon dynamics during tropical dry forest regeneration in Yucatán, Mexico. Ecosystems 7, 311–319 (2004).
Santiago, L. S. et al. Tropical tree seedling growth responses to nitrogen, phosphorus and potassium addition. J. Ecol. 100, 309–316 (2012).
Carpenter, A. T., Moore, J. C., Redente, E. F. & Stark, J. C. Plant community dynamics in a semi-arid ecosystem in relation to nutrient addition following a major disturbance. Plant Soil 126, 91–99 (1990).
Vitousek, P. M., Walker, L. R., Whiteaker, L. D. & Matson, P. A. Nutrient limitations to plant growth during primary succession in Hawaii Volcanoes National Park. Biogeochemistry 23, 197–215 (1993).
Batterman, S. A., Wurzburger, N. & Hedin, L. O. Nitrogen and phosphorus interact to control tropical symbiotic N2 fixation: a test in Inga punctata. J. Ecol. 101, 1400–1408 (2013).
Limpens, J., Berendse, F. & Klees, H. How phosphorus availability affects the impact of nitrogen deposition on sphagnum and vascular plants in bogs. Ecosystems 7, 793–804 (2004).
Zamin, T. J., Bret-Harte, M. S. & Grogan, P. Evergreen shrubs dominate responses to experimental summer warming and fertilization in Canadian mesic low arctic tundra. J. Ecol. 102, 749–766 (2014).
Lammerts, E. J., Pegtel, D. M., Grootjans, A. P. & van der Veen, A. Nutrient limitation and vegetation changes in a coastal dune slack. J. Veg. Sci. 10, 111–122 (1999).
Zhu, F., Lu, X., Mo, J. & EJ, P. Phosphorus limitation on photosynthesis of two dominant understory species in a lowland tropical forest. J. Plant Ecol. 7, 526–534 (2014).
Vitousek, P. M. & Farrington, H. Nutrient limitation and soil development: experimental test of a biogeochemical theory. Biogeochemistry 37, 63–75 (1997).
Augustine, D. J., McNaughton, S. J. & Frank, D. A. Feedbacks between soil nutrients and large herbivors in a managed savanna ecosystem. Ecol. Appl. 13, 1325–1337 (2003).
Alvarez, R., Santanatoglia, O. J. & Garcia, R. Effect of temperature on soil microbial biomass and its metabolic quotient in situ under different tillage systems. Biol. Fertil. Soils 19, 227–230 (1995).
Chen, F. -S., Zeng, D. -H., Fahey, T. J., Yao, C. -Y. & Yu, Z. -Y. Response of leaf anatomy of Chenopodium acuminatum to soil resource availability in a semi-arid grassland. Plant Ecol. 209, 375–382 (2010).
Mayor, J. R., Mack, M. C. & Schuur, E. A. G. Decoupled stoichiometric, isotopic, and fungal responses of an ectomycorrhizal black spruce forest to nitrogen and phosphorus additions. Soil. Biol. Biochem. 88, 247–256 (2015).
Van Duren, I. C., Boeye, D. & Grootjans, A. P. Nutrient limitations in an extant and drained poor fen: implications for restoration. Plant Ecol. 133, 91–100 (1997).
Haag, R. W. Nutrient limitations to plant production in two tundra communities. Can. J. Bot. 52, 103–116 (1974).
Sundqvist, M. K., Liu, Z., Giesler, R. & Wardle, D. A. Plant and microbial responses to nitrogen and phosphorus addition across an elevational gradient in subarctic tundra. Ecology 95, 1819–1835 (2014).
van der Hoek, D., van Mierlo Anita, J. E. M. & van Groenendael, J. M. Nutrient limitation and nutrient-driven shifts in plant species composition in a species-rich fen meadow. J. Veg. Sci. 15, 389–396 (2004).
Bowman, W. D., Theodose, T. A., Schardt, J. C. & Conant, R. T. Constraints of nutrient availability on primary production in two alpine tundra communities. Ecology 74, 2085–2097 (1993).
Davidson, E. A. et al. Nitrogen and phosphorus limitation of biomass growth in a tropical secondary forest. Ecol. Appl. 14, 150–163 (2004).
Güsewell, S., Koerselman, W. & Verhoeven, J. T. A. Time-dependent effects of fertilization on plant biomass in floating fens. J. Veg. Sci. 13, 705–718 (2002).
Ngai, J. T. & Jefferies, R. L. Nutrient limitation of plant growth and forage quality in Arctic coastal marshes. J. Ecol. 92, 1001–1010 (2004).
Potthast, K., Hamer, U. & Makeschin, F. In an Ecuadorian pasture soil the growth of Setaria sphacelata, but not of soil microorganisms, is co-limited by N and P. Appl. Soil Ecol. 62, 103–114 (2012).
Johnson, N. C., Wilson, G. W. T., Wilson, J. A., Miller, R. M. & Bowker, M. A. Mycorrhizal phenotypes and the Law of the Minimum. New Phytol. 205, 1473–1484 (2015).
Barger, N. N., D’Antonio, C. M., Ghneim, T., Brink, K. & Cuevas, E. Nutrient limitation to primary productivity in a secondary savanna in Venezuela. Biotropica 34, 493 (2002).
Soudzilovskaia, N. A., Onipchenko, V. G., Cornelissen, J. H. C. & Aerts, R. Biomass production, N/P ratio and nutrient limitation in a Caucasian alpine tundra plant community. J. Veg. Sci. 16, 399–406 (2005).
Gill, R. A. et al. Linking community and ecosystem development on Mount St Helens. Oecologia 148, 312–324 (2006).
Craine, J. M., Morrow, C. & Stock, W. D. Nutrient concentration ratios and co-limitation in South African grasslands. New Phytol. 179, 829–836 (2008).
von Oheimb, G. et al. N/P ratio and the nature of nutrient limitation in Calluna-dominated heathlands. Ecosystems 13, 317–327 (2010).
Iversen, C. M., Bridgham, S. D. & Kellogg, L. E. Scaling plant nitrogen use and uptake efficiencies in response to nutrient addition in peatlands. Ecology 91, 693–707 (2010).
Laliberté, E. et al. Experimental assessment of nutrient limitation along a 2-million-year dune chronosequence in the south-western Australia biodiversity hotspot. J. Ecol. 100, 631–642 (2012).
Onipchenko, V. G. et al. Alpine plant functional group responses to fertiliser addition depend on abiotic regime and community composition. Plant Soil 357, 103–115 (2012).
Fisher, J. B. et al. Nutrient limitation in rainforests and cloud forests along a 3,000-m elevation gradient in the Peruvian Andes. Oecologia. 172, 889–902 (2013).
Cusell, C., Kooijman, A. & Lamers, L. P. M. Nitrogen or phosphorus limitation in rich fens? Edaphic differences explain contrasting results in vegetation development after fertilization. Plant Soil 384, 153–168 (2014).
Zhan, S., Wang, Y., Zhu, Z., Li, W. & Bai, Y. Nitrogen enrichment alters plant N/P stoichiometry and intensifies phosphorus limitation in a steppe ecosystem. Environ. Exp. Bot. 134, 21–32 (2017).
Tischer, A. et al. Above- and below-ground linkages of a nitrogen and phosphorus co-limited tropical mountain pasture system—responses to nutrient enrichment. Plant Soil 391, 333–352 (2015).
He, M. & Dijkstra, F. A. Phosphorus addition enhances loss of nitrogen in a phosphorus-poor soil. Soil. Biol. Biochem. 82, 99–106 (2015).
Chen, F. -S. et al. Nitrogen and phosphorus additions alter nutrient dynamics but not resorption efficiencies of Chinese fir leaves and twigs differing in age. Tree. Physiol. 35, 1106–1117 (2015).
Alvarez-Clare, S. & Mack, M. C. Do foliar, litter, and root nitrogen and phosphorus concentrations reflect nutrient limitation in a lowland tropical wet forest? PLoS ONE 10, e0123796 (2015).
Homeier, J. et al. Tropical Andean forests are highly susceptible to nutrient inputs—rapid effects of experimental N and P addition to an Ecuadorian montane forest. PLoS ONE 7, e47128 (2012).
Dai, X., Ouyang, Z., Li, Y. & Wang, H. Variation in yield gap induced by nitrogen, phosphorus and potassium fertilizer in North China Plain. PLoS ONE 8, e82147 (2013).
R Development Core Team, R. & R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 1, 409 (2014).
Poisot, T. The digitize package: extracting numerical data from scatterplots. R J. 3, 25–26 (2011).
Maherali, H., Oberle, B., Stevens, P. F., Cornwell, W. K. & McGlinn, D. J. Mutualism persistence and abandonment during the evolution of the mycorrhizal symbiosis. Am. Nat. 188, E113–E125 (2016).
Viechtbauer, W. Conducting meta-analyses in R with the metafor. J. Stat. Softw. 36, 1–48 (2010).
Del Re, A. C. & Hoyt, W. T. MAd: meta-analysis with mean differences v.0.8-2 (CRAN, 2014); http://cran.r-project.org/web/packages/MAd
Acknowledgements
This study was supported by the International Program CryoCARB (MSM 7E10073—CryoCARB, Austrian Science Fund (FWF): I370-B17, German Federal Ministry of Education and Research (03F0616A)), project no. GA17-15229S and the SoWa Research Infrastructure funded by MEYS CZ grants LM2015075 and EF16_013/0001782—SoWa Ecosystems Research. S.M. acknowledges support from the Swedish Research Councils, Formas (2015-468) and VR (2016-04146) and the Bolin Centre for Climate Research. J.B., T.U. and H.S. were also supported by Czech Science Foundation project no. 16-18453 S. G.H. acknowledges the Joint Partnership Initiative project COUP and the Swedish Research Council grant no. E0689701 and the project CryoN funded by Academy of Finland (no. 132045). P.C. would also like to thank TES program of the U.S. Department of Energy (DOE) Office of Science, Biological and Environmental Research (BER) for partial support at Pacific Northwest National Laboratory (PNNL). PNNL is operated by Battelle for DOE. X. Xu kindly shared his dataset on microbial biomass elemental composition. We also thank N. Hess and B. Bond-Lamberty for comments and language corrections to this manuscript.
Author information
Authors and Affiliations
Contributions
P.C. collected data for meta-analysis and wrote the manuscript. P.C., S.M. and H.S. developed the conceptual framework. Other co-authors conducted a thorough critical review of the manuscript and contributed to manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Table 1
List of studies used in the meta-analysis with corresponding variables extracted from each study (ecosystem type, measured plant characteristic, soil N/P and C/N ratio, microbial critical N/P and C/N ratio, plant critical N/P ratio and dominant plant–microbe relationship).
Statistical meta-analysis
Step-by-step statistical meta-analysis with detailed additional information.
Rights and permissions
About this article
Cite this article
Čapek, P., Manzoni, S., Kaštovská, E. et al. A plant–microbe interaction framework explaining nutrient effects on primary production. Nat Ecol Evol 2, 1588–1596 (2018). https://doi.org/10.1038/s41559-018-0662-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-018-0662-8
This article is cited by
-
Response of Soil Microbial Communities and Nutrient Stoichiometry to Stand Age in Chinese Fir Plantations: Insights at the Aggregate Scale
Journal of Soil Science and Plant Nutrition (2024)
-
From guest to host: parasite Cistanche deserticola shapes and dominates bacterial and fungal community structure and network complexity
Environmental Microbiome (2023)
-
A lower labile C input relieves the negative effects of N enrichment on plant assemblages in a semi-arid grassland
Plant and Soil (2023)
-
Plant above-ground biomass and litter quality drive soil microbial metabolic limitations during vegetation restoration of subtropical forests
Soil Ecology Letters (2023)
-
Nitrogen enrichment enhances the competition for nitrogen uptake between Stipa purpurea and microorganisms in a tibetan alpine steppe
Plant and Soil (2023)