Abstract
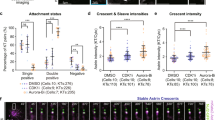
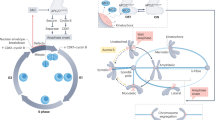
Loss of spindle-pole integrity during mitosis leads to multipolarity independent of centrosome amplification1,2,3,4. Multipolar-spindle conformation favours incorrect kinetochore–microtubule attachments, compromising faithful chromosome segregation and daughter-cell viability5,6. Spindle-pole organization influences and is influenced by kinetochore activity7,8, but the molecular nature behind this critical force balance is unknown. CLASPs are microtubule-, kinetochore- and centrosome-associated proteins whose functional perturbation leads to three main spindle abnormalities: monopolarity, short spindles and multipolarity9,10,11,12,13. The first two reflect a role at the kinetochore–microtubule interface through interaction with specific kinetochore partners10,11,14, but how CLASPs prevent spindle multipolarity remains unclear. Here we found that human CLASPs ensure spindle-pole integrity after bipolarization in response to CENP-E- and Kid-mediated forces from misaligned chromosomes. This function is independent of end-on kinetochore–microtubule attachments and involves the recruitment of ninein to residual pericentriolar satellites. Distinctively, multipolarity arising through this mechanism often persists through anaphase. We propose that CLASPs and ninein confer spindle-pole resistance to traction forces exerted during chromosome congression, thereby preventing irreversible spindle multipolarity and aneuploidy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Brenner, S., Branch, A., Meredith, S. & Berns, M. W. The absence of centrioles from spindle poles of rat kangaroo (PtK2) cells undergoing meiotic-like reduction division in vitro. J. Cell Biol. 72, 368–379 (1977).
Gergely, F., Draviam, V. M. & Raff, J. W. The ch-TOG/XMAP215 protein is essentialfor spindle pole organization in human somatic cells. Genes Dev. 17, 336–341 (2003).
Cassimeris, L. & Morabito, J. TOGp, the human homolog of XMAP215/Dis1, is required for centrosome integrity, spindle pole organization, and bipolar spindle assembly. Mol. Biol. Cell 15, 1580–1590 (2004).
Keryer, G., Ris, H. & Borisy, G. G. Centriole distribution during tripolar mitosis in Chinese hamster ovary cells. J. Cell Biol. 98, 2222–2229 (1984).
Ganem, N. J., Godinho, S. A. & Pellman, D. A mechanism linking extra centrosomes to chromosomal instability. Nature 460, 278–282 (2009).
Silkworth, W. T., Nardi, I. K., Scholl, L. M. & Cimini, D. Multipolar spindle pole coalescence is a major source of kinetochore mis-attachment and chromosome mis-segregation in cancer cells. PLoS ONE 4, e6564 (2009).
Gordon, M. B., Howard, L. & Compton, D. A. Chromosome movement inmitosis requires microtubule anchorage at spindle poles. J. Cell Biol. 152, 425–434 (2001).
Manning, A. L. & Compton, D. A. Mechanisms of spindle-pole organization are influenced by kinetochore activity in mammalian cells. Curr. Biol. 17, 260–265 (2007).
Maiato, H. et al. Human CLASP1 is an outer kinetochore component that regulates spindle microtubule dynamics. Cell 113, 891–904 (2003).
Maiato, H., Khodjakov, A. & Rieder, C. L. Drosophila CLASP is required for the incorporation of microtubule subunits into fluxing kinetochore fibres. Nat. Cell Biol. 7, 42–47 (2005).
Maffini, S. et al. Motor-independent targeting of CLASPs to kinetochores by CENP-E promotes microtubule turnover and poleward flux. Curr. Biol. 19, 1566–1572 (2009).
Pereira, A. L. et al. Mammalian CLASP1 and CLASP2 cooperate to ensure mitotic fidelity by regulating spindle and kinetochore function. Mol. Biol. Cell 17, 4526–4542 (2006).
Mimori-Kiyosue, Y. et al. Mammalian CLASPs are required for mitotic spindle organization and kinetochore alignment. Genes Cells 11, 845–857 (2006).
Manning, A. L. et al. CLASP1, astrin and Kif2b form a molecular switch that regulates kinetochore–microtubule dynamics to promote mitotic progression and fidelity. EMBO J. 29, 3531–3543 (2010).
Thein, K. H., Kleylein-Sohn, J., Nigg, E. A. & Gruneberg, U. Astrin is required for the maintenance of sister chromatid cohesion and centrosome integrity. J. Cell Biol. 178, 345–354 (2007).
McGuinness, B. E., Hirota, T., Kudo, N. R., Peters, J. M. & Nasmyth, K. Shugoshin prevents dissociation of cohesin from centromeres during mitosis in vertebrate cells. PLoS Biol. 3, e86 (2005).
Daum, J. R. et al. Cohesion fatigue induces chromatid separation in cells delayed at metaphase. Curr. Biol. 21, 1018–1024 (2011).
Stevens, D., Gassmann, R., Oegema, K. & Desai, A. Uncoordinated loss of chromatid cohesion is a common outcome of extended metaphase arrest. PLoS ONE 6, e22969 (2011).
Kwiatkowski, N. et al. Small-molecule kinase inhibitors provide insight into Mps1 cell cycle function. Nat. Chem. Biol. 6, 359–368 (2010).
Magidson, V. et al. The spatial arrangement of chromosomes during prometaphase facilitates spindle assembly. Cell 146, 555–567 (2011).
Cimini, D., Wan, X., Hirel, C. B. & Salmon, E. D. Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr. Biol. 16, 1711–1718 (2006).
Levesque, A. A. & Compton, D. A. The chromokinesin Kid is necessary for chromosome arm orientation and oscillation, but not congression, on mitotic spindles. J. Cell Biol. 154, 1135–1146 (2001).
Levesque, A. A., Howard, L., Gordon, M. B. & Compton, D. A. A functional relationship between NuMA and kid is involved in both spindle organization and chromosome alignment in vertebrate cells. Mol. Biol. Cell 14, 3541–3552 (2003).
Kapoor, T. M. et al. Chromosomes can congress to the metaphase plate before biorientation. Science 311, 388–391 (2006).
Cai, S., O’Connell, C. B., Khodjakov, A. & Walczak, C. E. Chromosome congression in the absence of kinetochore fibres. Nat. Cell Biol. 11, 832–838 (2009).
Sardar, H. S., Luczak, V. G., Lopez, M. M., Lister, B. C. & Gilbert, S. P. Mitotic kinesin CENP-E promotes microtubule plus-end elongation. Curr. Biol. 20, 1648–1653 (2010).
Wood, K. W. et al. Antitumor activity of an allosteric inhibitor of centromere-associated protein-E. Proc. Natl Acad. Sci. USA 107, 5839–5844 (2010).
Compton, D. A. Focusing on spindle poles. J. Cell Sci. 111, 1477–1481 (1998).
Garrett, S., Auer, K., Compton, D. A. & Kapoor, T. M. hTPX2 is required for normal spindle morphology and centrosome integrity during vertebrate cell division. Curr. Biol. 12, 2055–2059 (2002).
Bornens, M. Centrosome composition and microtubule anchoring mechanisms. Curr. Opin. Cell Biol. 14, 25–34 (2002).
Bettencourt-Dias, M. & Glover, D. M. Centrosome biogenesis and function: Centrosomics brings new understanding. Nat. Rev. 8, 451–463 (2007).
Dammermann, A. & Merdes, A. Assembly of centrosomal proteins and microtubule organization depends on PCM-1. J. Cell Biol. 159, 255–266 (2002).
Gruber, J., Harborth, J., Schnabel, J., Weber, K. & Hatzfeld, M. The mitotic-spindle-associated protein astrin is essential for progression through mitosis. J. Cell Sci. 115, 4053–4059 (2002).
Kim, K. & Rhee, K. The pericentriolar satellite protein CEP90 is crucial for integrity of the mitotic spindle pole. J. Cell Sci. 124, 338–347 (2011).
Oshimori, N., Li, X., Ohsugi, M. & Yamamoto, T. Cep72 regulates the localization of key centrosomal proteins and proper bipolar spindle formation. EMBO J. 28, 2066–2076 (2009).
Oshimori, N., Ohsugi, M. & Yamamoto, T. The Plk1 target Kizuna stabilizesmitotic centrosomes to ensure spindle bipolarity. Nat. Cell Biol. 8, 1095–1101 (2006).
Mattiuzzo, M. et al. Abnormal kinetochore-generated pulling forces from expressing a N-terminally modified Hec1. PLoS ONE 6, e16307 (2011).
Kubo, A., Sasaki, H., Yuba-Kubo, A., Tsukita, S. & Shiina, N. Centriolar satellites: Molecular characterization, ATP-dependent movement toward centrioles and possible involvement in ciliogenesis. J. Cell Biol. 147, 969–980 (1999).
Tokai-Nishizumi, N., Ohsugi, M., Suzuki, E. & Yamamoto, T. The chromokinesin Kid is required for maintenance of proper metaphase spindle size. Mol. Biol. Cell 16, 5455–5463 (2005).
Pereira, A. J. & Maiato, H. Improved kymography tools and its applications to mitosis. Methods 51, 214–219 (2010).
Acknowledgements
The authors would like to thank J. Macedo and P. Sampaio for technical help, A. Pereira for expertise in statistical analysis and all colleagues that provided invaluable reagents. E.L. is supported by Programa Ciência funded by Programa Operacional Potencial Humano (POPH)/QREN, as well as grant PTDC/SAU-OBD/100261/2008 from Fundação para a Ciência e a Tecnologia of Portugal (COMPETE-FEDER). S.M. held a fellowship from the Fundação para a Ciência e a Tecnologia (FCT) of Portugal (SFRH/BPD/26780/2006). P.M. holds an SNF-Professorship and a EURYI award. Work in the laboratory of H.M. is financially supported by grants PTDC/SAU-GMG/099704/2008 and PTDC/SAU-ONC/112917/2009 from Fundação para a Ciência e a Tecnologia of Portugal (COMPETE-FEDER), the Human Frontier Research Program and the seventh framework programme grant PRECISE from the European Research Council.
Author information
Authors and Affiliations
Contributions
E.L. designed, carried out and analysed most experiments. S.M. and A.M. carried out initial phenotypic characterization of CLASP1/2 RNAi. M.B. carried out flux measurements. A.T. and P.M. provided the EGFP–centrin-2/α-tubulin–mRFP stable HeLa cell line. H.M. designed experiments, analysed the data, coordinated the work and wrote the manuscript with contributions from E.L. and S.M.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 723 kb)
Supplementary Movie 1
Supplementary Information (MOV 1093 kb)
Supplementary Movie 2
Supplementary Information (MOV 4271 kb)
Supplementary Movie 3
Supplementary Information (MOV 2983 kb)
Supplementary Movie 4
Supplementary Information (MOV 718 kb)
Supplementary Movie 5
Supplementary Information (MOV 404 kb)
Supplementary Movie 6
Supplementary Information (MOV 1340 kb)
Supplementary Movie 7
Supplementary Information (MOV 1151 kb)
Supplementary Movie 8
Supplementary Information (MOV 2261 kb)
Supplementary Movie 9
Supplementary Information (MOV 562 kb)
Supplementary Movie 10
Supplementary Information (MOV 2046 kb)
Rights and permissions
About this article
Cite this article
Logarinho, E., Maffini, S., Barisic, M. et al. CLASPs prevent irreversible multipolarity by ensuring spindle-pole resistance to traction forces during chromosome alignment. Nat Cell Biol 14, 295–303 (2012). https://doi.org/10.1038/ncb2423
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2423
This article is cited by
-
Mild replication stress causes premature centriole disengagement via a sub-critical Plk1 activity under the control of ATR-Chk1
Nature Communications (2023)
-
Chromosomally unstable tumor cells specifically require KIF18A for proliferation
Nature Communications (2021)
-
SOGA1 and SOGA2/MTCL1 are CLASP-interacting proteins required for faithful chromosome segregation in human cells
Chromosome Research (2021)
-
Mild replication stress causes chromosome mis-segregation via premature centriole disengagement
Nature Communications (2019)
-
Selective autophagy maintains centrosome integrity and accurate mitosis by turnover of centriolar satellites
Nature Communications (2019)