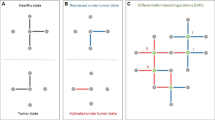
Abstract
The formation of protein complexes and the co-regulation of the cellular concentrations of proteins are essential mechanisms for cellular signaling and for maintaining homeostasis. Here we use isobaric-labeling multiplexed proteomics to analyze protein co-regulation and show that this allows the identification of protein–protein associations with high accuracy. We apply this 'interactome mapping by high-throughput quantitative proteome analysis' (IMAHP) method to a panel of 41 breast cancer cell lines and show that deviations of the observed protein co-regulations in specific cell lines from the consensus network affects cellular fitness. Furthermore, these aberrant interactions serve as biomarkers that predict the drug sensitivity of cell lines in screens across 195 drugs. We expect that IMAHP can be broadly used to gain insight into how changing landscapes of protein–protein associations affect the phenotype of biological systems.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Aebersold, R. & Mann, M. Mass-spectrometric exploration of proteome structure and function. Nature 537, 347–355 (2016).
Rolland, T. et al. A proteome-scale map of the human interactome network. Cell 159, 1212–1226 (2014).
Huttlin, E.L. et al. The BioPlex network: a systematic exploration of the human interactome. Cell 162, 425–440 (2015).
Hein, M.Y. et al. A human interactome in three quantitative dimensions organized by stoichiometries and abundances. Cell 163, 712–723 (2015).
McAlister, G.C. et al. Increasing the multiplexing capacity of TMTs using reporter ion isotopologues with isobaric masses. Anal. Chem. 84, 7469–7478 (2012).
Heiser, L.M. et al. Subtype- and pathway-specific responses to anticancer compounds in breast cancer. Proc. Natl. Acad. Sci. USA 109, 2724–2729 (2012).
Ting, L., Rad, R., Gygi, S.P. & Haas, W. MS3 eliminates ratio distortion in isobaric multiplexed quantitative proteomics. Nat. Methods 8, 937–940 (2011).
McAlister, G.C. et al. MultiNotch MS3 enables accurate, sensitive and multiplexed detection of differential expression across cancer cell line proteomes. Anal. Chem. 86, 7150–7158 (2014).
Neve, R.M. et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 10, 515–527 (2006).
Zhang, B. et al. Proteogenomic characterization of human colon and rectal cancer. Nature 513, 382–387 (2014).
Klijn, C. et al. A comprehensive transcriptional portrait of human cancer cell lines. Nat. Biotechnol. 33, 306–312 (2015).
Szklarczyk, D. et al. The STRING database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 39, D561–D568 (2011).
Wang, J. et al. Proteome profiling outperforms transcriptome profiling for co-expression-based gene function prediction. Mol. Cell. Proteomics http://dx.doi.org/10.1074/mcp.M116.060301 (2016).
Stefely, J.A. et al. Mitochondrial protein functions elucidated by multi-omic mass spectrometry profiling. Nat. Biotechnol. 34, 1191–1197 (2016).
Chick, J.M. et al. Defining the consequences of genetic variation on a proteome-wide scale. Nature 534, 500–505 (2016).
Ruepp, A. et al. CORUM: the comprehensive resource of mammalian protein complexes—2009. Nucleic Acids Res. 38, D497–D501 (2010).
Heath, C.G., Viphakone, N. & Wilson, S.A. The role of TREX in gene expression and disease. Biochem. J. 473, 2911–2935 (2016).
Goldberg, A.L. & Dice, J.F. Intracellular protein degradation in mammalian and bacterial cells. Annu. Rev. Biochem. 43, 835–869 (1974).
Marcotte, R. et al. Functional genomic landscape of human breast cancer drivers, vulnerabilities and resistance. Cell 164, 293–309 (2016).
Berger, A.H. et al. High-throughput phenotyping of lung cancer somatic mutations. Cancer Cell 30, 214–228 (2016).
Lawrence, M.S. et al. Discovery and saturation analysis of cancer genes across 21 tumor types. Nature 505, 495–501 (2014).
Nik-Zainal, S. et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature 534, 47–54 (2016).
Huang, W., Sherman, B.T. & Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Ioannidis, S. et al. Discovery of 5-chloro-N2-[(1S)-1-(5-fluoropyrimidin-2-yl)ethyl]-N4-(5-methyl-1H-pyrazol-3-yl)pyrimidine-2,4-diamine (AZD1480) as a novel inhibitor of the Jak–Stat pathway. J. Med. Chem. 54, 262–276 (2011).
Jester, B.W., Gaj, A., Shomin, C.D., Cox, K.J. & Ghosh, I. Testing the promiscuity of commercial kinase inhibitors against the AGC kinase group using a split-luciferase screen. J. Med. Chem. 55, 1526–1537 (2012).
Davis, M.I. et al. Comprehensive analysis of kinase inhibitor selectivity. Nat. Biotechnol. 29, 1046–1051 (2011).
O'Hare, T. et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell 16, 401–412 (2009).
El-Mir, M.Y. et al. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J. Biol. Chem. 275, 223–228 (2000).
Miyadera, H. et al. Atpenins, potent and specific inhibitors of mitochondrial complex II (succinate–ubiquinone oxidoreductase). Proc. Natl. Acad. Sci. USA 100, 473–477 (2003).
Kagawa, Y. & Racker, E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. 8. Properties of a factor conferring oligomycin sensitivity on mitochondrial adenosine triphosphatase. J. Biol. Chem. 241, 2461–2466 (1966).
Dephoure, N. et al. Quantitative proteomic analysis reveals posttranslational responses to aneuploidy in yeast. eLife 3, e03023 (2014).
Stingele, S. et al. Global analysis of genome, transcriptome and proteome reveals the response to aneuploidy in human cells. Mol. Syst. Biol. 8, 608 (2012).
Mertins, P. et al. Proteogenomics connects somatic mutations to signaling in breast cancer. Nature 534, 55–62 (2016).
Villén, J. & Gygi, S.P. The SCX/IMAC enrichment approach for global phosphorylation analysis by mass spectrometry. Nat. Protoc. 3, 1630–1638 (2008).
Haas, W. et al. Optimization and use of peptide mass measurement accuracy in shotgun proteomics. Mol. Cell. Proteomics 5, 1326–1337 (2006).
Wessel, D. & Flügge, U.I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 138, 141–143 (1984).
Edwards, A. & Haas, W. Multiplexed quantitative proteomics for high-throughput comprehensive proteome comparisons of human cell lines. Methods Mol. Biol. 1394, 1–13 (2016).
Thompson, A. et al. Tandem mass tags: a novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Anal. Chem. 75, 1895–1904 (2003).
Wang, Y. et al. Reversed-phase chromatography with multiple fraction concatenation strategy for proteome profiling of human MCF10A cells. Proteomics 11, 2019–2026 (2011).
Huttlin, E.L. et al. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell 143, 1174–1189 (2010).
Eng, J.K., McCormack, A.L. & Yates, J.R.I. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 5, 976–989 (1994).
Peng, J., Elias, J.E., Thoreen, C.C., Licklider, L.J. & Gygi, S.P. Evaluation of multidimensional chromatography coupled with tandem mass spectrometry (LC/LC-MS/MS) for large-scale protein analysis: the yeast proteome. J. Proteome Res. 2, 43–50 (2003).
Elias, J.E., Haas, W., Faherty, B.K. & Gygi, S.P. Comparative evaluation of mass spectrometry platforms used in large-scale proteomics investigations. Nat. Methods 2, 667–675 (2005).
Elias, J.E. & Gygi, S.P. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat. Methods 4, 207–214 (2007).
R Developmental Core Team. R: a language and environment for statistical computing. (R Foundation for Statistical Computing, Vienna, Austria) (2011).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Marcotte, R. et al. Essential gene profiles in breast, pancreatic and ovarian cancer cells. Cancer Discov. 2, 172–189 (2012).
Garnett, M.J. et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature 483, 570–575 (2012).
Tong, P. et al. drexplorer: a tool to explore dose–response relationships and drug–drug interactions. Bioinformatics 31, 1692–1694 (2015).
Huang, D.W. et al. DAVID bioinformatics resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 35, W169–W175 (2007).
Acknowledgements
We thank S. Gygi (Harvard Medical School) for access to computational software and facilities to process the proteomics data. We are grateful to J. Boisvert for her help with culturing of the cell lines and to all members of the Haas and Benes laboratories for valuable discussions. C.H.B. is supported by grant 102696 from the Wellcome Trust. Cell lines were purchased with funds from the NIH LINCS phase 1 grant HG006097.
Author information
Authors and Affiliations
Contributions
C.H.B. and W.H. conceived and designed the study; J.D.L., C.H.B. and W.H. wrote the manuscript; J.D.L. and W.H. performed the proteomics experiments; P.G. and C.H.B. performed the drug screen and analyzed the drug screen data; and J.D.L., R.M., A.A., I.P.-M., C.H.B. and W.H. performed the analysis of the proteomics data.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Spearman’s rank correlation matrix of the proteome profiles of 41 breast cancer cell lines.
Data are listed in Supplementary Tables 1 and 2.
Supplementary Figure 2 Hierarchical clustering of 36 breast cancer cell lines based on their mRNA or protein profiles.
Hierarchical clustering of 36 breast cancer cell lines based on their (a) mRNA or (b) protein profiles. Clustering was done for the 36 cell lines, which proteome profiles were mapped in this study and which mRNA profiles were also reported by Klinj et al.1 Although the dendrograms show differences in the composition of the sub-clusters, the clustering based both on mRNA and protein profiles generated similar clusters of cell lines of the basal (green), luminal (red), claudin-low (blue), and non-malignant (brown) subtypes. Unsupervised clustering was done based on the intensities of 6659 gene-products quantified in all datasets and using the JMP software (version Pro 11) applying the ward method without standardizing the data.
1. Klijn, C. et al. A comprehensive transcriptional portrait of human cancer cell lines. Nat Biotechnol 33, 306-312, doi:10.1038/nbt.3080 (2015).
Supplementary Figure 3 Global comparison of protein and mRNA levels for 36 breast cancer cell lines.
(a) Scatterplot of protein levels measured in two biological replicates of HCC1937 proteomes. Protein levels were calculated as the log2 ratio of the protein level determined in each duplicates over the median protein level in all proteome measurements (duplicates of 36 cell lines); log2 HCC1937 intensity/median intensity. (b) Scatterplot of protein levels measured in one biological duplicate versus mRNA level measured by RNA sequencing analysis. Both levels are given as log2 HCC1937 intensity/median intensity. (c) Spearman correlation distribution for duplicate proteomics measurements (dark blue, median Spearman’s r = 0.82) and mRNA and protein level comparisons (yellow, median ρ = 0.62).
Supplementary Figure 4 Protein co-regulation analysis identifies substantially more known protein–protein associations than mRNA co-regulation analysis across a range mRNA of precision thresholds.
The analysis was done on identical numbers of gene products monitored in 36 cell lines. The RNAseq data published by Klinj et al.1 were used for mRNA-based co-regulation analysis. High confidence known protein-protein associations were extracted from the STRING database (score ≥ 0.700)2. The co-regulation analysis is described in the Supplementary Methods Section.
1. Klijn, C. et al. A comprehensive transcriptional portrait of human cancer cell lines. Nat Biotechnol 33, 306-312, doi:10.1038/nbt.3080 (2015).
2. Szklarczyk, D. et al. The STRING database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res 39, D561-568, doi:10.1093/nar/gkq973 (2011).
Supplementary Figure 5 Validating novel protein–protein associations found through protein co-regulation analysis using extended STRING database matching.
A novel association between proteins A and B is confirmed in the extended STRING database match if the association between protein B and another protein C detected in our study to also being associated with protein A is confirmed in the STRING database.
Supplementary Figure 6 Comparison of co-regulation data with AP-MS interactome maps to identify protein complexes.
Even the conservative cut-off of an FDR of 0.05 % used in our manuscript produces a set of protein-protein associations with 71 % not listed in the CORUM database, a conservatively curated database of known protein complexes. It should be noted that this compares favorably to the two prominent AP-MS studies on mapping the human interactome1,2. Only 6 % of the 23,744 interactions reported by Huttlin et al.1 are listed in the CORUM database and 7 % of the 31,944 interactions presented by Hein et al.2. We have compared our dataset with these AP-MS datasets. For this comparison we only considered associations, where both protein partners were identified in both datasets. This was done to remove experimental bias regarding the identified proteins. The number of associations from our dataset fulfilling this requirement was 8,128, out of which 678 (8 %) were also reported by Huttlin et al.. Out of the 678 associations 501 (74 %) are CORUM listed and 177 (26 %) are novel interactions. The percentage of known interactions in our sub-dataset was 35 % (2,825 associations) and 501 of those (18 %) were confirmed by Huttlin et al., while the 177 unknown interactions confirmed by Huttlin et al. constituted 3 % of the 5,303 unknown associations in our dataset. In summary, the comparison shows that associations found in both dataset are enriched for known associations but that unknown associations was also confirmed by the AP-MS interactome mapping. To put this comparison into perspective, we also compared another AP-MS mapping performed by Hein et al. with that of Huttlin et al. in the exactly same manner. 16,357 associations reported by Hein et al. fulfilled the requirement of both protein-partners being identified in interactions in both datasets. 6 % (998) of them were confirmed by Huttlin et al., of which 390 (39 %) were known and 61 % (608) were novel. Of all the known interactions in the Hein et al. sub-dataset (1,758), 22 % (390) were also found by Huttlin et al.. The overlap was 4 % (608 out of 16357) for the unknown interactions. In summary, we show that novel associations in our dataset are confirmed by AP-MS data and that our dataset compares with AP-MS data in a similar way that AP-MS datasets compare with each other.
1. Huttlin, E. L. et al. The BioPlex Network: A Systematic Exploration of the Human Interactome. Cell 162, 425-440, doi:10.1016/j.cell.2015.06.043 (2015).
2. Hein, M. Y. et al. A human interactome in three quantitative dimensions organized by stoichiometries and abundances. Cell 163, 712-723, doi:10.1016/j.cell.2015.09.053 (2015).
Supplementary Figure 7 Dysregulated protein–protein associaction networks in basal-subtype breast cancer cell lines.
Proteins with dysregulated protein-protein associations are shown in red on the entire network of all protein-protein associations defined through co-regulation analysis (Fig. 2, Supplementary Table 6). Association dysregulations were determined by bivariate outlier detection of deviations of co-regulation of any protein pair across 41 breast cancer cell lines calculating the Mahalnobis distance and using the Grubb’s outlier test (p<0.1, Supplementary Table 8).
Supplementary Figure 8 Dysregulated protein–protein associaction networks in luminal-subtype breast cancer cell lines.
Proteins with dysregulated protein-protein associations are shown in red on the entire network of all protein-protein associations defined through co-regulation analysis (Fig. 2, Supplementary Table 6). Association dysregulations were determined by bivariate outlier detection of deviations of co-regulation of any protein pair across 41 breast cancer cell lines calculating the Mahalnobis distance and using the Grubb’s outlier test (p<0.1, Supplementary Table 8).
Supplementary Figure 10 Gene copy number correlations correlate strongly with mRNA but not with protein profiles, explaining the difference in the derived functional gene-association networks.
(a) Protein and mRNA profile Spearman’s correlation matrices of 32 members of the 26S proteasome multi-protein complex (Supplementary Table 13). Protein co-regulation identifies 107 associations across the members and the correlation matrix reflects the proteasome substructure. Co-regulation of mRNA profiles identifies 3 high confidence interactions across the proteasome members. (b) Gene copy number variations are strongly correlated with mRNA levels of proteasome members (median ρ = 0.55, right panel) but do not correlated with protein levels (median ρ = -0.01, left panel).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 (PDF 1584 kb)
Supplementary Tables
Supplementary Tables 1–13 (ZIP 85252 kb)
Supplementary Data
Supplementary data 1–3 (ZIP 1098 kb)
Rights and permissions
About this article
Cite this article
Lapek, J., Greninger, P., Morris, R. et al. Detection of dysregulated protein-association networks by high-throughput proteomics predicts cancer vulnerabilities. Nat Biotechnol 35, 983–989 (2017). https://doi.org/10.1038/nbt.3955
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.3955
This article is cited by
-
Mapping protein states and interactions across the tree of life with co-fractionation mass spectrometry
Nature Communications (2023)
-
A computational framework for the inference of protein complex remodeling from whole-proteome measurements
Nature Methods (2023)
-
PARP1-SNAI2 transcription axis drives resistance to PARP inhibitor, Talazoparib
Scientific Reports (2022)
-
iDRiP for the systematic discovery of proteins bound directly to noncoding RNA
Nature Protocols (2021)
-
Pan-cancer proteogenomic investigations identify post-transcriptional kinase targets
Communications Biology (2021)