Abstract
The maternally inherited symbiotic Wolbachia have been previously shown to have much greater densities in insecticide-resistant Culex pipiens mosquitoes than in insecticide-susceptible individuals. These high densities were shown to be at least partially responsible for the costs related to insecticide resistance in this species. We report here the rapid evolution, on the order of 50 generations, of bacterial densities both in laboratory and field populations. Along with other recently published studies, this report shows that Wolbachia–host interactions are very dynamic.
Similar content being viewed by others
Introduction
Wolbachia are maternally inherited symbiotic bacteria, which manipulate their host's reproduction, either by modifying host sex ratio or by inducing cytoplasmic incompatibilities (Werren, 1997; Charlat et al., 2003). Moreover, they have sometimes, but not always (for example, Poinsot and Mercot, 1997; Vavre et al., 2002), been shown to have deleterious effects on their host's life history traits and fitness (Min and Benzer, 1997; McGraw et al., 2002; Mouton et al., 2004; Weeks et al., 2007).
In the mosquito Culex pipiens, these bacteria cause cytoplasmic incompatibility (CI) where early embryo death results from crosses between infected males and uninfected females, or from crosses between males and females infected by incompatible bacterial strains (Yen and Barr, 1973; Duron et al., 2006a). It was recently shown that mosquitoes resistant to organophosphate insecticides have higher Wolbachia loads than insecticide-susceptible mosquitoes (Berticat et al., 2002) and that these higher loads are accompanied by deleterious effects on host life history traits, such as, preimaginal mortality, adult body size and fecundity (Duron et al., 2006c). These observations were interpreted as resulting from a deregulation of control over bacterial replication in insecticide-resistant genomic backgrounds.
Recent studies on Drosophila–Wolbachia interactions showed that the virulence of these maternally transmissible symbionts can decrease very rapidly both in laboratory and field populations (McGraw et al., 2002; Weeks et al., 2007). McGraw and colleagues showed that virulence attenuation was accompanied by a decrease in bacterial density.
The aim of this study was to investigate whether a similar phenomenon occurred in insecticide-resistant C. pipiens mosquitoes. To this end we analyzed Wolbachia density in the same strains that Berticat et al. (2002) analyzed 36 generations previously. We also measured bacterial densities in insecticide-susceptible and -resistant mosquitoes from the same field population as Berticat et al. (2002), approximately 55 generations after their study. Our results indicate that within these relatively short time intervals Wolbachia densities in insecticide-resistant mosquitoes have evolved to levels equivalent to those of susceptible mosquitoes, both in the lab and in the field.
Materials and methods
Mosquitoes
We used the same three strains of C. pipiens as used by Berticat et al. (2002): S-LAB, SA4 and SR. The S-LAB strain (Georghiou et al., 1966) is an insecticide-susceptible strain. SA4 and SR are insecticide-resistant strains with an S-LAB nuclear and cytoplasmic (including Wolbachia) genetic background and homozygous for the resistance alleles Ester4 at the Ester locus and ace-1R at the ace-1 locus, respectively (for details on the strains, see Berticat et al., 2002). We measured Wolbachia densities of both insecticide-resistant strains and compared them with those of the insecticide-susceptible strain, S-LAB. We analyzed 65 S-LAB females and 82 males, 70 SA4 females and 93 males, 76 SR females and 56 males 36 generations after Berticat et al. (2002), and asked whether Wolbachia densities had evolved in this time interval. The insecticide resistance of strain SR is periodically tested through biochemical assays and its resistance properties have not evolved (M Weill, personal observation).
We also sampled in September 2005 mosquitoes from the same field site, situated at Ganges (43°55′02.08″N; 3°42′43.06″E), near Montpellier (France), that Berticat et al. (2002) had sampled in July 2001. This site is not treated by insecticides. As in Berticat et al. (2002), pupae and larvae isolated from the field were raised in the lab under standard conditions (25 °C and 70% humidity, given food ad libitum, with larval densities less than 1000 larvae per liter and with a water depth less than 2 cm) until 5-day-old adults were obtained. Using the same protocols as Berticat et al. (2002) we characterized insecticide resistance at the Ester and at the ace-1 loci. The only difference in protocols was that instead of using an enzymatic bioassay to characterize resistance at the ace-1 locus as Berticat et al. (2002), we used a PCR/RFLP test (Weill et al., 2004). Using these protocols, we screened 120 individuals and isolated from the field sample two types of individuals: (1) insecticide-susceptible (S) and (2) insecticide-resistant mosquitoes carrying the Ester4 allele but not the ace-1R allele (A4). We then estimated Wolbachia densities from 12 S male, 12 S female, 11 A4 male and 7 A4 female individuals and compared the Wolbachia load of the September 2005 samples to their July 2001 counterparts.
Estimating Wolbachia density
Bacterial density was estimated by real-time quantitative PCR using an Applied Biosystem 7300 machine (same machine as in Berticat et al., 2002). All estimates were taken from 5-day-old male and female adults to standardize comparisons as Wolbachia density is known to vary with both mosquito gender and age (Berticat et al., 2002). The experimental procedures were identical to those of Berticat et al. (2002). In short, two PCRs were performed on each mosquito's DNA: one specific for the Culex ace-2 locus (Weill et al., 2000), which is not involved in insecticide resistance and the other specific for the Wolbachia wsp locus (Berticat et al., 2002). To obtain accurate estimates of the gene copy number, we plotted standard curves using dilutions of a pBluescriptKS vector containing one copy each of the ace-2 and wsp fragments. The same dilutions as in the study of Berticat et al. (2002) were used to construct the standards.
Each DNA template was analyzed in duplicate or triplicate for wsp and ace-2 quantification. The ratio between wsp and ace-2 arbitrary concentrations provided the number of Wolbachia genomes relative to Culex genomes, thus correcting for mosquito size and DNA extraction quality.
To assess the quality of our qPCRs we analyzed the efficiency and the repeatability of our measurements by considering amplification efficiency (AE) and the repeatability between replicates. Amplification efficiency represents the multiplication of PCR product increase during each cycle, which is between 1 and 2. The Percentile amplification efficiency (PAE) represents the percentage of full AE capacity, which is between 0 and 100%. AE and PAE are calculated through standard curves for Ct or fluorescent signal strength during the amplification process. A linear fit with a slope approximately between −3.1 and −3.6, equivalent to 90–110% reaction efficiency, is typically acceptable for most applications requiring accurate quantification. In our study the average slope is −3.50 (s.d. 0.21) and the PAE is on average 93.44% (s.d. 7.09).
We assessed the repeatability of the measurements by comparing the Ct values of replicates of each sample. We calculated the mean Ct value, the standard deviation and the coefficient of variation for each sample. The median coefficient of variation was 0.00282 (2.5th percentile: 0.00015; 97.5th percentile: 0.02811) for lab-reared mosquitoes and 0.00064 (2.5th percentile: 0.000001; 97.5th percentile: 0.02552) for field mosquitoes.
Claire Berticat (ISEM, Montpellier, France) provided data from the Berticat et al. (2002) study.
Statistical analysis
Data on Wolbachia densities were analyzed using fully factorial models, with insecticide resistance genotype (S-LAB, SA4 or SR for laboratory strains, S or A4 for field samples) and mosquito gender as fixed factors. As standard assumptions of analysis of variance were not met we used the nonparametric Scheirer-Ray-Hare extension of the Kruskal–Wallis test (H statistic; see Sokal and Rohlf, 2003, pp 446–447). Sums of squares based on rank transformed data were computed using JMP (SAS Institute, Cary, NC, USA).
Results
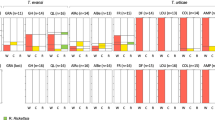
Figure 1 summarizes our results. It is important to keep in mind that although Figure 1 exhibits Wolbachia densities, all the tests were performed on ranks.
Wolbachia density in laboratory strains and field samples. We present results for the samples analyzed by Berticat et al. (2002) (‘old’) and in this study (‘new’). For each genotype/time of observation/gender/origin of samples, we present the individual ratios of Wolbachia/mosquito genome counts (black dots) and the median of each distribution (black squares). To increase the clarity of the figure we only present individuals whose ratio <12. The following individuals are thus not represented: (1) one SR old lab male with a ratio ∼30; (2) one SA4 old lab female—ratio ∼15; (3) three S new field females—two with ratio ∼30 and one with ratio ∼20; (iv) two A4 new field females—one with ratio ∼40 and one with ratio ∼20. Please note that the figure shows the ratios whereas the tests mentioned in the text bear on their relative ranks.
Wolbachia density in lab strains
Across all strains, females have roughly 10 times more Wolbachia than males of the same age (1.388 vs 0.1068 Wolbachia mosquito−1 genomes; H=186.66, d.f.=1, P<10−6). Contrary to Berticat et al. (2002), we found that S-LAB mosquitoes had the same Wolbachia load as resistant mosquitoes (H=0.61, P=0.7371). The interaction between sex and strain was significant (H=10.60, P=0.005), in that S-LAB females harbored relatively more Wolbachia than insecticide-resistant females, whereas S-LAB males harbored relatively less Wolbachia than insecticide-resistant males.
As we estimated, Wolbachia densities using exactly the same procedures as Berticat et al. (2002), it was possible to combine data from both studies to analyze bacterial density with a model including the factors strain, sex and time (‘old’ vs ‘new’). As Figure 1 shows, overall Wolbachia densities decreased over time (‘old’ vs ‘new’ comparison; H=55.52, P<10−6), the decrease being mostly due to change in the resistant strains. Mosquito sex was the only other factor with a significant effect on Wolbachia densities (H=151.6, P<10−6).
The effects of factors strain and the interaction strain × time were close to the significance threshold (P=0.0626 and P=0.0792). As the differences between the two sexes are substantial, we performed separate analyses for each sex. We found that in females the strain effect was not significant (H=1.19, d.f.=2, P=0.5515), whereas the time and time × strain interactions were highly significant (time: H=47.48, d.f.=1, P<10−6; time × strain: H=23.88, d.f.=2, P<10−6). In males, the strain effect (H=11.54, d.f.=2, P=0.003) and the time effects were significant (H=52, d.f.=1, P<10−6) but the time × strain interaction was not (H=0.45, d.f.=2, P=0.7985). In brief, the above statistical tests lead to the following observations: (1) for both males and females, the Wolbachia density of insecticide-susceptible individuals was similar in the ‘old’ and ‘new’ samples; (2) for both males and females, ‘old’ insecticide-resistant mosquitoes had higher Wolbachia densities than ‘new’ mosquitoes; furthermore, as reported by Berticat et al. (2002), in the ‘old’ sample for both males and females insecticide-resistant individuals had higher Wolbachia densities than insecticide-susceptible individuals, whereas in the ‘new’ sample Wolbachia densities did not differ with respect to the insecticide resistance genotypes.
Wolbachia density in field samples
The analysis of the field samples revealed similar results to that of the lab strains: females harbored 30 times more Wolbachia than males (H=23.78, P<0.0001), but there were no differences in Wolbachia load according to insecticide resistance genotype (H=0.16, P=0.69). The interaction between gender and insecticide resistance genotype is not significant (H=0.016, P=0.9).
The comparison of ‘old’ and ‘new’ samples also revealed similar results: the full factorial analysis, including factors genotype (S vs A4), gender and time (‘old’ vs ‘new’), revealed a very strong gender effect (females having much higher Wolbachia densities than males; H=42.92, d.f.=1, P<10−6) and a time effect (overall Wolbachia density has decreased in the field; H=4.846, d.f.=1, P=0.028), all other factors having very little effect (associated P values>0.35) except for the time × genotype interaction (H=2.068, d.f.=1, P=0.15). Because of the very strong gender effect, we repeated the analysis separately for males and females. We found that in males the ‘new’ samples had lower Wolbachia densities (H=14.41, d.f.=1, P=0.0001) but Wolbachia densities were not affected by the insecticide resistance genotype (H=0.892, d.f.=1, P=0.3449) nor the interaction time × genotype (H=0.1469). In females, only the time × genotype interaction had a marginally significant effect (genotype: H=0.06, d.f.=1, P=0.8064; time: H=0.97, d.f.=1, P=0.3246; time × genotype: H=3.48, d.f.=1, P=0.0621), indicating that although Wolbachia densities remained the same in insecticide-susceptible field females they decreased in A4 females.
Discussion
As in previous studies involving several species (for example, C. pipiens (Berticat et al., 2002); Aedes albopictus (Dobson et al., 1999); Drosophila simulans (Bourtzis et al., 1998); two planthopper species (Noda et al., 2001)) we found a higher Wolbachia load in females than in males. This gender difference could be due to several factors. As proposed by Berticat et al. (2002) it could simply result from an organ size effect. Another explanation derives from their mode of transmission: Wolbachia are vertically transmitted through the eggs. Wolbachia in male mosquitoes participate in the invasion of the host population by these bacteria through the phenomenon of CI. However, there is no evidence of a link between CI intensity and Wolbachia density in C. pipiens (Duron et al., 2006c).
Like Berticat et al., we found higher Wolbachia densities in females sampled in the field than in the laboratory strains. This comparison, however, is not very meaningful as Wolbachia density is known to depend not only on host and symbiont genotypes but also on the environment in which individuals grow (for example, Mouton et al., 2006, 2007). The fact that larval environment is not controlled for the field samples must be at least partially responsible for the higher variance we observed. Another likely factor contributing to this higher variation in the field is the potential greater variation in Wolbachia and mosquito genotypes. For example, this population contained in 2001 at least 10 different Wolbachia genotypes (Duron et al., 2006b).
The most interesting aspect of our results concerns the evolution of Wolbachia density over time. Berticat et al. (2002) showed that insecticide-resistant strains, sharing the same cytoplasmic and nuclear background with an insecticide-susceptible strain, harbored much higher Wolbachia loads. An analysis of field samples confirmed this result. This difference was interpreted as being due to a less efficient control of Wolbachia load by insecticide-resistant mosquitoes, which could result from physiological costs associated with insecticide resistance genes. The higher Wolbachia density, in the extent that it is correlated to insecticide resistance, could feedback and increase the physiological costs of insecticide resistance. This was indeed reported by Duron et al. (2006c) on the same lab strains as in Berticat et al. (2002) analyzed 12 generations after the original density measurements by Berticat et al. (2002).
As Wolbachia are vertically inherited, it is expected that their deleterious effects should decrease over time. This was indeed observed in Drosophila both in the laboratory (McGraw et al., 2002; Weeks et al., 2007) and in the field (Weeks et al., 2007). If these negative effects are correlated with Wolbachia density, we would expect the bacterial load to evolve to lower levels, and such a decrease of bacterial density was indeed observed in laboratory Drosophila populations (McGraw et al., 2002). Our results show that Wolbachia density evolved in the insecticide-resistant laboratory strains within a maximum of 36 generations, whereas it remained constant in the insecticide-susceptible strain (it is worth noting that the fact that Wolbachia densities do not differ between ‘old’ and ‘new’ insecticide susceptible samples a posteriori corroborates our use of the qPCR technique to estimate and compare microbial densities). The analysis of the field samples corroborated this finding. Within the 50 months separating the two sampling points, roughly corresponding to 55 generations (13 generations per year (Lenormand et al., 1999)), the Wolbachia load of insecticide-resistant mosquitoes had evolved to levels comparable to those of insecticide-susceptible mosquitoes. The relative rate of evolution of Wolbachia loads in the laboratory vs field populations would depend on (1) whether the rate of the process is mostly limited by the rate of de novo mutations or by the strength of selection; (2) the control of Wolbachia density (host factors, bacterial factors, interaction between the two). It is highly likely that evolution is mostly limited by de novo mutations in the laboratory, because all strains share the same cytoplasmic host and bacterial backgrounds as well as most of the nuclear genomic background. Moreover, in laboratory populations a given cytoplasmic background is confronted with the same nuclear background in each generation. This is not the case in field populations, which are polymorphic for insecticide resistance, where a given cytoplasm may alternate between insecticide-resistant and -susceptible nuclear genomic backgrounds over generations. The strength of selection in favor of Wolbachia density control mechanisms is thus probably weaker in the field, although, because of the huge population densities and high migration rates that these mosquitoes exhibit, mutation is probably less limiting.
We cannot tell whether the rapid evolution we observe is due to changes in the host or the bacterial genome, or to coevolution between the two. Weeks et al. (2007) were able to demonstrate that in their case evolution of the bacteria was responsible for the observed changes. Future studies are needed to elucidate the mechanisms underlying the rapid evolution of Wolbachia density in insecticide-resistant mosquitoes, as well as to verify that the decrease in density has resulted in a decrease of associated physiological costs. Nevertheless, this study, in agreement with the studies on Wolbachia in Drosophila (McGraw et al., 2002; Weeks et al., 2007) and in a nymphalid butterfly (Hornett et al., 2006; Charlat et al., 2007), demonstrates that host–Wolbachia interactions can evolve extremely rapidly.
References
Berticat C, Rousset F, Raymond M, Berthomieu A, Weill M (2002). High Wolbachia density in insecticide-resistant mosquitoes. Proc Biol Sci 269: 1413–1416.
Bourtzis K, Dobson SL, Braig HR, O'Neill SL (1998). Rescuing Wolbachia have been overlooked. Nature 391: 852–853.
Charlat S, Hornett EA, Fullard JH, Davies N, Roderick GK, Wedell N et al. (2007). Extraordinary flux in sex ratio. Science 317: 214.
Charlat S, Hurst GD, Mercot H (2003). Evolutionary consequences of Wolbachia infections. Trends Genet 19: 217–223.
Dobson SL, Bourtzis K, Braig HR, Jones BF, Zhou W, Rousset F et al. (1999). Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochem Mol Biol 29: 153–160.
Duron O, Bernard C, Unal S, Berthomieu A, Berticat C, Weill M (2006a). Tracking factors modulating cytoplasmic incompatibilities in the mosquito Culex pipiens. Mol Ecol 15: 3061–3071.
Duron O, Fort P, Weill M (2006b). Hypervariable prophage WO sequences describe an unexpected high number of Wolbachia variants in the mosquito Culex pipiens. Proc Biol Sci 273: 495–502.
Duron O, Labbe P, Berticat C, Rousset F, Guillot S, Raymond M et al. (2006c). High Wolbachia density correlates with cost of infection for insecticide resistant Culex pipiens mosquitoes. Evolution 60: 303–314.
Georghiou GP, Metcalf RL, Gidden FE (1966). Carbamate-resistance in mosquitos. Selection of Culex pipiens fatigans Wiedemann (=C. quinquefasciatus Say) for resistance to Baygon. Bull World Health Organ 35: 691–708.
Hornett EA, Charlat S, Duplouy AM, Davies N, Roderick GK, Wedell N et al. (2006). Evolution of male-killer suppression in a natural population. PLoS Biol 4: e283.
Lenormand T, Bourguet D, Guillemaud T, Raymond M (1999). Tracking the evolution of insecticide resistance in the mosquito Culex pipiens. Nature 400: 861–864.
McGraw EA, Merritt DJ, Droller JN, O'Neill SL (2002). Wolbachia density and virulence attenuation after transfer into a novel host. Proc Natl Acad Sci USA 99: 2918–2923.
Min KT, Benzer S (1997). Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc Natl Acad Sci USA 94: 10792–10796.
Mouton L, Dedeine F, Henri H, Bouletreau M, Profizi N, Vavre F (2004). Virulence multiple infections and regulation of symbiotic population in the Wolbachia-Asobara tabida symbiosis. Genetics 168: 181–189.
Mouton L, Henri H, Bouletreau M, Vavre F (2006). Effect of temperature on Wolbachia density and impact on cytoplasmic incompatibility. Parasitology 132: 49–56.
Mouton L, Henri H, Charif D, Bouletreau M, Vavre F (2007). Interaction between host genotype and environmental conditions affects bacterial density in Wolbachia symbiosis. Biol Lett 3: 210–213.
Noda H, Koizumi Y, Zhang Q, Deng K (2001). Infection density of Wolbachia and incompatibility level in two planthopper species, Laodelphax striatellus and Sogatella furcifera. Insect Biochem Mol Biol 31: 727–737.
Poinsot D, Mercot H (1997). Wolbachia infection in Drosophila simulans: does the female host bear a physiological cost? Evolution 51: 180–186.
Sokal RR, Rohlf FS (2003). Biometry: 3rd Edition. WH Freeman and Company: New York, NY.
Vavre F, Fleury F, Varaldi J, Fouillet P, Bouletreau M (2002). Infection polymorphism and cytoplasmic incompatibility in Hymenoptera-Wolbachia associations. Heredity 88: 361–365.
Weeks AR, Turelli M, Harcombe WR, Reynolds KT, Hoffmann AA (2007). From parasite to mutualist: rapid evolution of Wolbachia in natural populations of Drosophila. PLoS Biol 5: 997–1005.
Weill M, Berticat C, Raymond M, Chevillon C (2000). Quantitative polymerase chain reaction to estimate the number of amplified esterase genes in insecticide-resistant mosquitoes. Anal Biochem 285: 267–270.
Weill M, Malcolm C, Chandre F, Mogensen K, Berthomieu A, Marquine M et al. (2004). The unique mutation in ace-1 giving high insecticide resistance is easily detectable in mosquito vectors. Insect Mol Biol 13: 1–7.
Werren JH (1997). Biology of Wolbachia. Annu Rev Entomol 42: 587–609.
Yen JH, Barr AR (1973). The etiological agent of cytoplasmic incompatibility in Culex pipiens. J Invertebr Pathol 22: 242–250.
Acknowledgements
We are grateful to Claire Berticat for providing us data, advice and comments on previous versions of the article. PE, PA, CS, VN and YM acknowledge funding from the CNRS and the IRD.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Echaubard, P., Duron, O., Agnew, P. et al. Rapid evolution of Wolbachia density in insecticide resistant Culex pipiens. Heredity 104, 15–19 (2010). https://doi.org/10.1038/hdy.2009.100
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hdy.2009.100
Keywords
This article is cited by
-
Genetic stability of Aedes aegypti populations following invasion by wMel Wolbachia
BMC Genomics (2021)
-
Characterization of bacterial communities associated with blood-fed and starved tropical bed bugs, Cimex hemipterus (F.) (Hemiptera): a high throughput metabarcoding analysis
Scientific Reports (2021)
-
First report of natural Wolbachia infection in the malaria mosquito Anopheles arabiensis in Tanzania
Parasites & Vectors (2018)
-
Tripartite associations among bacteriophage WO, Wolbachia, and host affected by temperature and age in Tetranychus urticae
Experimental and Applied Acarology (2012)
-
Many compatible Wolbachia strains coexist within natural populations of Culex pipiens mosquito
Heredity (2011)