Abstract
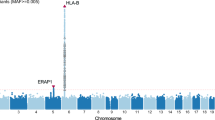
Acute anterior uveitis (AAU) involves inflammation of the iris and ciliary body of the eye. It occurs both in isolation and as a complication of ankylosing spondylitis (AS). It is strongly associated with HLA-B*27, but previous studies have suggested that further genetic factors may confer additional risk. We sought to investigate this using the Illumina Exomechip microarray, to compare 1504 cases with AS and AAU, 1805 with AS but no AAU and 21 133 healthy controls. We also used a heterogeneity test to test the differences in effect size between AS with AAU and AS without AAU. In the analysis comparing AS+AAU+ cases versus controls, HLA-B*27 and HLA-A*02:01 were significantly associated with the presence of AAU (P<10−300 and P=6 × 10−8, respectively). Secondary independent association with PSORS1C3 (P=4.7 × 10−5) and TAP2 (P=1.1 × 10−5) were observed in the major histocompatibility complex. There was a new suggestive association with a low-frequency variant at zinc-finger protein 154 in the AS without AAU versus control analysis (zinc-finger protein 154 (ZNF154), P=2.2 × 10−6). Heterogeneity testing showed that rs30187 in ERAP1 has a larger effect on AAU compared with that in AS alone. These findings also suggest that variants in ERAP1 have a differential impact on the risk of AAU when compared with AS, and hence the genetic risk for AAU differs from AS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 digital issues and online access to articles
$119.00 per year
only $19.83 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Robinson PC, Brown MA . Genetics of ankylosing spondylitis. Mol Immunol 2014; 57: 2–11.
Robinson PC, Claushuis TA, Cortes A, Martin TM, Evans DM, Leo P et al. Genetic dissection of acute anterior uveitis reveals similarities and differences in associations observed with ankylosing spondylitis. Arthritis Rheumatol 2015; 67: 140–151.
Derhaag PJ, Linssen A, Broekema N, de Waal LP, Feltkamp TE . A familial study of the inheritance of HLA-B27-positive acute anterior uveitis. Am J Ophthalmol 1988; 105: 603–606.
Pennesi G, Caspi RR . Genetic control of susceptibility in clinical and experimental uveitis. Int Rev Immunol 2002; 21: 67–88.
Tennessen JA, Bigham AW, O'Connor TD, Fu W, Kenny EE, Gravel S et al. Evolution and functional impact of rare coding variation from deep sequencing of human exomes. Science 2012; 337: 64–69.
Silverberg MS, Cho JH, Rioux JD, McGovern DP, Wu J, Annese V et al. Ulcerative colitis—risk loci on chromosomes 1p36 and 12q15 found by genome-wide association study. Nat Genet 2009; 41: 216–220.
Raelson JV, Little RD, Ruether A, Fournier H, Paquin B, Van Eerdewegh P et al. Genome-wide association study for Crohn's disease in the Quebec Founder Population identifies multiple validated disease loci. Proc Natl Acad Sci USA 2007; 104: 14747–14752.
Evans DM, Spencer CC, Pointon JJ, Su Z, Harvey D, Kochan G et al. Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat Genet 2011; 43: 761–767.
Cortes A, Pulit SL, Leo PJ, Pointon JJ, Robinson PC, Weisman MH et al. Major histocompatibility complex associations of ankylosing spondylitis are complex and involve further epistasis with ERAP1. Nat Commun 2015; 6: 7146.
Cortes A, Hadler J, Pointon JP, Robinson PC, Karaderi T, Leo P et al. Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat Genet 2013; 45: 730–738.
Zeboulon N, Dougados M, Gossec L . Prevalence and characteristics of uveitis in the spondyloarthropathies: a systematic literature review. Ann Rheum Dis 2008; 67: 955–959.
Robinson PC, Costello ME, Leo P, Bradbury LA, Hollis K, Cortes A et al. ERAP2 is associated with ankylosing spondylitis in HLA-B27-positive and HLA-B27-negative patients. Ann Rheum Dis 2015; 74: 1627–1629.
Kenna TJ, Lau MC, Keith P, Ciccia F, Costello M-E, Bradbury L et al. Disease-associated polymorphisms in ERAP1 do not alter endoplasmic reticulum stress in patients with ankylosing spondylitis. Genes Immunity 2015; 16: 35–42.
Robinson PC, Lau E, Keith P, Lau MC, Thomas GP, Bradbury LA et al. ERAP2 functional knockout in humans does not alter surface heavy chains or HLA-B27, inflammatory cytokines or endoplasmic reticulum stress markers. Ann Rheum Dis 2015; 74: 2092–2095.
Wucherpfennig KW . Mechanisms for the induction of autoimmunity by infectious agents. J Clin Invest 2001; 108: 1097–1104.
Robinson PC, Brown MA . The genetics of ankylosing spondylitis and axial spondyloarthritis. Rheum Dis Clin North Am 2012; 38: 539–553.
Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature Working G.. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol 2005; 140: 509–516.
van der Linden S, Valkenburg HA, Cats A . Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984; 27: 361–368.
Goldstein JI, Crenshaw A, Carey J, Grant GB, Maguire J, Fromer M et al. zCall: a rare variant caller for array-based genotyping: genetics and population analysis. Bioinformatics 2012; 28: 2543–2545.
Jia X, Han B, Onengut-Gumuscu S, Chen WM, Concannon PJ, Rich SS et al. Imputing amino acid polymorphisms in human leukocyte antigens. PLoS One 2013; 8: e64683.
Peloso GM, Auer PL, Bis JC, Voorman A, Morrison AC, Stitziel NO et al. Association of low-frequency and rare coding-sequence variants with blood lipids and coronary heart disease in 56,000 whites and blacks. Am J Hum Genet 2014; 94: 223–232.
Wu MC, Lee S, Cai T, Li Y, Boehnke M, Lin X . Rare-variant association testing for sequencing data with the sequence kernel association test. Am J Hum Genet 2011; 89: 82–93.
Price AL, Kryukov GV, de Bakker PI, Purcell SM, Staples J, Wei LJ et al. Pooled association tests for rare variants in exon-resequencing studies. Am J Hum Genet 2010; 86: 832–838.
Madsen BE, Browning SR . A groupwise association test for rare mutations using a weighted sum statistic. PLoS Genet 2009; 5: e1000384.
Morris AP, Zeggini E . An evaluation of statistical approaches to rare variant analysis in genetic association studies. Genet Epidemiol 2010; 34: 188–193.
Li B, Leal SM . Methods for detecting associations with rare variants for common diseases: application to analysis of sequence data. Am J Hum Genet 2008; 83: 311–321.
Lee S, Wu MC, Lin X . Optimal tests for rare variant effects in sequencing association studies. Biostatistics 2012; 13: 762–775.
Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P et al. A method and server for predicting damaging missense mutations. Nat Methods 2010; 7: 248–249.
Acknowledgements
We acknowledge all patients who donated their DNA. PR was funded by the National Health and Medical Research Council (NHMRC) of Australia and is currently funded by the ARA-RACP-Starr Research Fellowship. MAB is supported by a Senior Principal Research Fellowship of the NHMRC. The AOGC ExomeChip genotyping was funded by NHMRC Project Grant 1032571 (ELD). The AOGC cohort collection was funded by NHMRC Project Grant 511132. Additional funding was provided by the NIHR Oxford Comprehensive Biomedical Research Centre (immunity and inflammation theme A93081), Arthritis Research UK (Grants 19536 and 18797), the NIHR Thames Valley collaborative research network and the National Ankylosing Spondylitis Society (UK).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Membership of Wellcome Trust Case Control Consortium 2 (WTCCC2) Management committee: Peter Donnelly (Chair)1,2, Ines Barroso (Deputy Chair)3, Jenefer M Blackwell4,5, Elvira Bramon6, Matthew A Brown7, Juan P Casas8, Aiden Corvin9, Panos Deloukas3, Audrey Duncanson10, Janusz Jankowski11, Hugh S Markus12, Christopher G Mathew13, Colin NA Palmer14, Robert Plomin15, Anna Rautanen1, Stephen J Sawcer16, Richard C Trembath13, Ananth C Viswanathan17, Nicholas W Wood18. Data and analysis group: Chris CA Spencer1, Gavin Band1, Céline Bellenguez1, Colin Freeman1, Garrett Hellenthal1, Eleni Giannoulatou1, Matti Pirinen1, Richard Pearson1, Amy Strange1, Zhan Su1, Damjan Vukcevic1, Peter Donnelly1,2. DNA, genotyping, data QC and informatics group: Cordelia Langford3, Sarah E Hunt3, Sarah Edkins3, Rhian Gwilliam3, Hannah Blackburn3, Suzannah J Bumpstead3, Serge Dronov3, Matthew Gillman3, Emma Gray3, Naomi Hammond3, Alagurevathi Jayakumar3, Owen T McCann3, Jennifer Liddle3, Simon C Potter3, Radhi Ravindrarajah3, Michelle Ricketts3, Matthew Waller3, Paul Weston3, Sara Widaa3, Pamela Whittaker3, Ines Barroso3, Panos Deloukas3. Publications committee: Christopher G Mathew (Chair)13, Jenefer M Blackwell4,5, Matthew A Brown7, Aiden Corvin9, Chris C A Spencer1 1Wellcome Trust Centre for Human Genetics, University of Oxford, Roosevelt Drive, Oxford OX3 7BN, UK; 2Department of Statistics, University of Oxford, Oxford OX1 3TG, UK; 3Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridge CB10 1SA, UK; 4Telethon Institute for Child Health Research, Centre for Child Health Research, University of Western Australia, 100 Roberts Road, Subiaco, WA 6008, Australia; 5Cambridge Institute for Medical Research, University of Cambridge School of Clinical Medicine, Cambridge CB2 0XY, UK; 6Department of Psychosis Studies, NIHR Biomedical Research Centre for Mental Health at the Institute of Psychiatry, King’s College London and The South London and Maudsley NHS Foundation Trust, Denmark Hill, London SE5 8AF, UK; 7University of Queensland Diamantina Institute, Brisbane, QLD, Australia; 8Department of Epidemiology and Population Health, London School of Hygiene and Tropical Medicine, London WC1E 7HT and Department of Epidemiology and Public Health, University College London WC1E 6BT, UK; 9Neuropsychiatric Genetics Research Group, Institute of Molecular Medicine, Trinity College Dublin, Dublin 2, Eire; 10Molecular and Physiological Sciences, The Wellcome Trust, London NW1 2BE, UK; 11Department of Oncology, Old Road Campus, University of Oxford, Oxford OX3 7DQ, UK, Digestive Diseases Centre, Leicester Royal Infirmary, Leicester LE7 7HH, UK and Centre for Digestive Diseases, Queen Mary University of London, London E1 2AD, UK; 12Clinical Neurosciences, St George’s University of London, London SW17 0RE, UK; 13King’s College London Department of Medical and Molecular Genetics, King’s Heath Partners, Guy’s Hospital, London SE1 9RT, UK; 14Biomedical Research Centre, Ninewells Hospital and Medical School, Dundee DD1 9SY, UK; 15King’s College, London Social, Genetic and Developmental Psychiatry Centre, Institute of Psychiatry, Denmark Hill, London SE5 8AF, UK; 16University of Cambridge Department of Clinical Neurosciences, Addenbrooke’s Hospital, Cambridge CB2 0QQ, UK; 17NIHR Biomedical Research Centre for Ophthalmology, Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology, London EC1V 2PD, UK; 18Department of Molecular Neuroscience, Institute of Neurology, Queen Square, London WC1N 3BG, UK.
Membership of Australasian Osteoporosis Genetics Consortium (AOGC) contributing to ExomeChip samples Eugene McCloskey1, John Eisman2, Graeme Jones3, Geoff Nicholson4, Richard Eastell5, Philip Sambrook6 (deceased), Richard Prince7, Elaine Dennison8, Ian Reid9, John Wark10 1Academic Unit of Bone Metabolism, University of Sheffield, UK; 2Garvan Institute of Medical Research, University of New South Wales, Australia; 3Menzies Research Institute, University of Tasmania, Australia; 4Rural Clinical School, University of Queensland, Australia; 5Academic Unit of Bone Metabolism, Metabolic Bone Centre, University of Sheffield, UK; 6Kolling Institute, Royal North Shore Hospital, University of Sydney, Australia; 7School of Medicine and Pharmacology, University of Western Australia, Australia; 8Medical Research Council Lifecourse Epidemiology Unit, University of Southampton, UK; 9Department of Medicine, University of Auckland, New Zealand; 10Department of Medicine, University of Melbourne, Australia.
Supplementary Information accompanies this paper on Genes and Immunity website
Supplementary information
Rights and permissions
About this article
Cite this article
Robinson, P., Leo, P., Pointon, J. et al. The genetic associations of acute anterior uveitis and their overlap with the genetics of ankylosing spondylitis. Genes Immun 17, 46–51 (2016). https://doi.org/10.1038/gene.2015.49
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gene.2015.49
This article is cited by
-
A large meta-analysis identifies genes associated with anterior uveitis
Nature Communications (2023)
-
Progress in the genetics of uveitis
Genes & Immunity (2022)
-
Correlation of serum amyloid A levels, clinical manifestations, treatment, and disease activity in patients with acute anterior uveitis
Eye (2020)
-
lncRNA PSORS1C3 is regulated by glucocorticoids and fine-tunes OCT4 expression in non-pluripotent cells
Scientific Reports (2019)