Abstract
Efficient duplication of the genome requires the concerted action of helicase and DNA polymerases at replication forks1 to avoid stalling of the replication machinery and consequent genomic instability2,3,4. In eukaryotes, the physical coupling between helicase and DNA polymerases remains poorly understood. Here we define the molecular mechanism by which the yeast Ctf4 protein links the Cdc45–MCM–GINS (CMG) DNA helicase to DNA polymerase α (Pol α) within the replisome. We use X-ray crystallography and electron microscopy to show that Ctf4 self-associates in a constitutive disk-shaped trimer. Trimerization depends on a β-propeller domain in the carboxy-terminal half of the protein, which is fused to a helical extension that protrudes from one face of the trimeric disk. Critically, Pol α and the CMG helicase share a common mechanism of interaction with Ctf4. We show that the amino-terminal tails of the catalytic subunit of Pol α and the Sld5 subunit of GINS contain a conserved Ctf4-binding motif that docks onto the exposed helical extension of a Ctf4 protomer within the trimer. Accordingly, we demonstrate that one Ctf4 trimer can support binding of up to three partner proteins, including the simultaneous association with both Pol α and GINS. Our findings indicate that Ctf4 can couple two molecules of Pol α to one CMG helicase within the replisome, providing a new model for lagging-strand synthesis in eukaryotes that resembles the emerging model for the simpler replisome of Escherichia coli5,6,7,8. The ability of Ctf4 to act as a platform for multivalent interactions illustrates a mechanism for the concurrent recruitment of factors that act together at the fork.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Primary accessions
Protein Data Bank
Data deposits
Coordinates and structure factors for Ctf4(CTD) (selenomethionine-labelled protein), Ctf4(CTD) (native), Ctf4(CTD)–Pol α and Ctf4(CTD)–Sld5 complexes are available from the Protein Data Bank under accession codes 4C8H, 4C8S, 4C93 and 4C95, respectively.
References
DePamphilis, M. L. & Bell, S. D. Genome Duplication (Garland Science, 2011)
Zegerman, P. & Diffley, J. F. X. DNA replication as a target of the DNA damage checkpoint. DNA Repair (Amst.) 8, 1077–1088 (2009)
Cortez, D. Unwind and slow down: checkpoint activation by helicase and polymerase uncoupling. Genes Dev. 19, 1007–1012 (2005)
Errico, A. & Costanzo, V. Mechanisms of replication fork protection: a safeguard for genome stability. Crit. Rev. Biochem. Mol. Biol. 47, 222–235 (2012)
Lia, G., Michel, B. N. D. & Allemand, J.-F. S. S. Polymerase exchange during Okazaki fragment synthesis observed in living cells. Science 335, 328–331 (2012)
Georgescu, R. E., Kurth, I. & O’Donnell, M. Single-molecule studies reveal the function of a third polymerase in the replisome. Nature Struct. Mol. Biol. 19, 113–116 (2012)
McInerney, P., Johnson, A., Katz, F. & O’Donnell, M. Characterization of a triple DNA polymerase replisome. Mol. Cell 27, 527–538 (2007)
Reyes-Lamothe, R., Sherratt, D. J. & Leake, M. C. Stoichiometry and architecture of active DNA replication machinery in Escherichia coli. Science 328, 498–501 (2010)
Tanaka, H. et al. Ctf4 coordinates the progression of helicase and DNA polymerase α. Genes Cells 14, 807–820 (2009)
Gambus, A. et al. A key role for Ctf4 in coupling the MCM2–7 helicase to DNA polymerase α within the eukaryotic replisome. EMBO J. 28, 2992–3004 (2009)
Sengupta, S. et al. Dpb2 integrates the leading-strand DNA polymerase into the eukaryotic replisome. Curr. Biol. 23, 543–552 (2013)
Lou, H. et al. Mrc1 and DNA polymerase ε function together in linking DNA replication and the S phase checkpoint. Mol. Cell 32, 106 (2008)
Zhu, W. et al. Mcm10 and And-1/CTF4 recruit DNA polymerase alpha to chromatin for initiation of DNA replication. Genes Dev. 21, 2288–2299 (2007)
Kouprina, N. et al. CTF4 (CHL15) mutants exhibit defective DNA metabolism in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 12, 5736–5747 (1992)
Miles, J. & Formosa, T. Evidence that POB1, a Saccharomyces cerevisiae protein that binds to DNA polymerase α, acts in DNA metabolism in vivo. Mol. Cell. Biol. 12, 5724–5735 (1992)
Gosnell, J. A. & Christensen, T. W. Drosophila Ctf4 is essential for efficient DNA replication and normal cell cycle progression. BMC Mol. Biol. 12, 13 (2011)
Bermudez, V. P., Farina, A., Tappin, I. & Hurwitz, J. Influence of the human cohesion establishment factor Ctf4/AND-1 on DNA replication. J. Biol. Chem. 285, 9493–9505 (2010)
Williams, D. & McIntosh, J. mcl1+, the Schizosaccharomyces pombe homologue of CTF4, is important for chromosome replication, cohesion, and segregation. Eukaryot. Cell 1, 758–773 (2002)
Tanaka, H. et al. Replisome progression complex links DNA replication to sister chromatid cohesion in Xenopus egg extracts. Genes Cells 14, 949–963 (2009)
Petronczki, M. et al. Sister-chromatid cohesion mediated by the alternative RF-CCtf18/Dcc1/Ctf8, the helicase Chl1 and the polymerase-α-associated protein Ctf4 is essential for chromatid disjunction during meiosis II. J. Cell Sci. 117, 3547–3559 (2004)
Hanna, J. S., Kroll, E. S., Lundblad, V. & Spencer, F. A. Saccharomyces cerevisiae CTF18 and CTF4 are required for sister chromatid cohesion. Mol. Cell. Biol. 21, 3144–3158 (2001)
Yoshizawa-Sugata, N. & Masai, H. Roles of human AND-1 in chromosome transactions in S phase. J. Biol. Chem. 284, 20718–20728 (2009)
Gambus, A. et al. GINS maintains association of Cdc45 with MCM in replisome progression complexes at eukaryotic DNA replication forks. Nature Cell Biol. 8, 358–366 (2006)
Costa, A. et al. The structural basis for MCM2–7 helicase activation by GINS and Cdc45. Nature Struct. Mol. Biol. 18, 471–477 (2011)
Chang, Y. P., Wang, G., Bermudez, V., Hurwitz, J. & Chen, X. S. Crystal structure of the GINS complex and functional insights into its role in DNA replication. Proc. Natl Acad. Sci. USA 104, 12685–12690 (2007)
Yardimci, H., Loveland, A. B., Habuchi, S., van Oijen, A. M. & Walter, J. C. Uncoupling of sister replisomes during eukaryotic DNA replication. Mol. Cell 40, 834–840 (2010)
Beattie, T. R. & Bell, S. D. Coordination of multiple enzyme activities by a single PCNA in archaeal Okazaki fragment maturation. EMBO J. 31, 1556–1567 (2012)
Kelley, L. A. & Sternberg, M. J. E. Protein structure prediction on the Web: a case study using the Phyre server. Nature Protocols 4, 363–371 (2009)
Peränen, J., Rikkonen, M., Hyvonen, M. & Kaariainen, L. T7 vectors with modified T7lac promoter for expression of proteins in Escherichia coli. Anal. Biochem. 236, 371–373 (1996)
Aslanidis, C. & de Jong, P. J. Ligation-independent cloning of PCR products (LIC-PCR). Nucleic Acids Res. 18, 6069–6074 (1990)
Rigaut, G. et al. A generic protein purification method for protein complex characterization and proteome exploration. Nature Biotechnol. 17, 1030–1032 (1999)
Van Duyne, G. D., Standaert, R. F., Karplus, P. A., Schreiber, S. L. & Clardy, J. Atomic structures of the human immunophilin FKBP-12 complexes with FK506 and rapamycin. J. Mol. Biol. 229, 105–124 (1993)
Kastner, B. et al. GraFix: sample preparation for single-particle electron cryomicroscopy. Nature Methods 5, 53–55 (2008)
Kabsch, W. Xds. Acta Crystallogr. D 66, 125–132 (2010)
Evans, P. R. An introduction to data reduction: space-group determination, scaling and intensity statistics. Acta Crystallogr. D 67, 282–292 (2011)
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010)
Emsley, P. & Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004)
Davis, I. W. et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 35, W375–W383 (2007)
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Cryst. 40, 658–674 (2007)
Tang, G. et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007)
Costa, A. et al. Structural studies of the archaeal MCM complex in different functional states. J. Struct. Biol. 156, 210–219 (2006)
van Heel, M., Harauz, G., Orlova, E. V., Schmidt, R. & Schatz, M. A new generation of the IMAGIC image processing system. J. Struct. Biol. 116, 17–24 (1996)
Kilkenny, M. L., De Piccoli, G., Perera, R. L., Labib, K. & Pellegrini, L. A conserved motif in the C-terminal tail of DNA polymerase α tethers primase to the eukaryotic replisome. J. Biol. Chem. 287, 23740–23747 (2012)
Hernández, H. & Robinson, C. V. Determining the stoichiometry and interactions of macromolecular assemblies from mass spectrometry. Nature Protocols 2, 715–726 (2007)
Pettersen, E. F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004)
Acknowledgements
We would like to thank L. Collinson and R. Carzaniga (LRI) for EM access; D. Frith and B. Snijder (LRI) for mass spectrometry work; J. Maman for help with SEC-MALS; and P. Zegerman and J. Gannon for comments on the manuscript. This work was supported by the Gates Cambridge PhD program (A.C.S.), CRUK (A.C. and K.L.), MRC (K.L.) and a Wellcome Trust SRF award in basic biomedical science (L.P.).
Author information
Authors and Affiliations
Contributions
A.C. and L.P. conceived the project; A.C.S., J.C.Z., R.L.P., K.L., A.C. and L.P. designed experiments; A.C.S., J.C.Z., R.L.P., C.E., F.v.D., M.E.I., M.L.K., L.R., S.K. and D.M.-V. performed experiments; A.C.S., J.C.Z., D.M.-V., K.L., A.C. and L.P. analysed the data; A.C. and L.P. wrote the paper, with contributions and critical comments from the other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
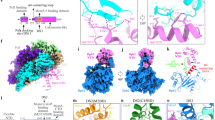
Extended Data Figure 1 Crystal structure of yeast Ctf4 region spanning amino acids 471–927 (C-end; Ctf4(CTD)).
a, The six-bladed β-propeller of Ctf4(CTD). The structure is drawn as a ribbon diagram, coloured blue to light green from the N to the C terminus. The six blades of the propeller are labelled WD1 to WD6, from the N to C end. The helical domain has been omitted for clarity. b, Two views of the Ctf4(CTD) protomer, highlighting the helical domain of Ctf4(CTD), coloured yellow to red from the amino to the carboxy terminus. The α-helices are labelled α1 to α6. The β-propeller domain is in light grey.
Extended Data Figure 2 2D EM of full-length Ctf4 and Ctf4(CTD).
a, Silver-stained SDS–PAGE gel showing the purified full-length yeast Ctf4 and GraFix gel of the same preparation. A red box highlights the fraction imaged by EM. b, Representative micrograph of the full-length yeast Ctf4. c, Zoomed-in view of the micrograph in panel b. d, Reference-free class averages of the full-length protein complex highlight the presence of a ring-shaped core linked to mobile, satellite densities. Box size 448 Å. e, Multi-angle light scattering reveals that Ctf4 forms stable homotrimers in solution (absolute molecular mass equates to 322 kDa, with a 0.6% error; expected molecular mass for a monomer: 106.9 kDa). f, Silver-stained SDS–PAGE gel showing the purified Ctf4(CTD) and GraFix gel of the same preparation. A red box highlights the fraction imaged by EM. g, Representative micrograph of the Ctf4(CTD) complex. h, Zoomed-in view of the micrograph in panel g. i, Reference-free class averages of the Ctf4(CTD) protein complex highlight the presence of an oligomerization core. Box size 448 Å.
Extended Data Figure 3 Crystal forms of the Ctf4(CTD) structure.
a, b, Side-by-side comparisons of the symmetric, closed form of the Ctf4(CTD) trimer (left-hand side) with the pseudo-symmetric, open form (right-hand side), used for study of the interaction with the Ctf4-binding motifs of Pol α and Sld5. Panel a shows a side view of the two crystal forms, drawn as ribbons, with the β-propeller domain of the three protomers coloured blue, cyan and light blue, respectively, and the helical domain coloured yellow. Panel b shows a top view of the two crystal forms, drawn as ribbons, with the symmetric, closed form of the Ctf4(CTD) trimer in light grey and the pseudo-symmetric, open form in dark grey. The helical domains have been removed for clarity. c–e, Superpositions of the Cα traces of the protomers of the symmetric, closed form of the Ctf4(CTD) trimer, the protomers of the pseudo-symmetric, open Ctf4(CTD) trimer and all protomers of the two crystal forms, respectively. In panels c and d, the protomers are coloured according to secondary structure, with β-strands in cyan and α-helices in yellow; the crystal form of the superimposed protomers is highlighted by a space-fill model of the structure in the top-left corner of the panel. In panel e, the Ctf4(CTD) protomers of the closed and open forms are coloured light and dark grey, respectively.
Extended Data Figure 4 Atomic details of the Ctf4–Pol α (a) and Ctf4–Sld5 (b) interfaces.
Ctf4(CTD) is drawn as a yellow ribbon, the Ctf4-binding motifs of Pol α and Sld5 as green and red tubes. The side chains of amino acids at the interface are shown as sticks, with carbon atoms coloured white (Ctf4) or light brown (Pol α and Sld5), oxygen atoms in red, nitrogen atoms in blue and sulphur atoms in yellow. The bidentate salt link between D142 (Pol1) or D7 (Sld5) and R904 of Ctf4 is shown as solid pink lines.
Extended Data Figure 5 Structure-based multiple sequence alignment of S. cerevisiae Ctf4, S. pombe Mcl1 and H. sapiens And1.
Only the region corresponding to the crystal structure described in the paper is reported. Observed secondary structure elements of Ctf4 and predicted secondary structure elements of Mcl1 and And1 are boxed and shaded in green and yellow for β-sheets and α-helices, respectively. The extent of the WD40 domains of the six-bladed β-propeller and the α-helices of the helical domain are illustrated above the alignment. Ctf4 residues that form the interface with Pol α and Sld5 are marked by an asterisk.
Extended Data Figure 6 Ctf4 interactions with Pol α and CMG.
a, Binding affinity of Ctf4(CTD) for the Ctf4-binding motifs of Pol α (top panel) and Sld5 (bottom panel). Affinity was measured by fluorescence anisotropy of fluorescein-labelled peptides in the presence of increasing amounts of Ctf4. See Methods for experimental details. b, CMG still associates with Ctf4 and Pol1 in yeast cells with mutations in the Ctf4-binding motif of Sld5. The budding yeast strains MCM4-5FLAG (Control) and MCM4-5FLAG sld5- Δ 2-9 (the endogenous copy of SLD5 was modified to create sld5- Δ 2-9, such that the encoded protein lacks amino acids 2-9) were grown at 24 °C, arrested in G1 phase, and then released into S phase for 30 min. Mcm4-5FLAG was then isolated from cell extracts by immunoprecipitation, and the indicated proteins were detected by immunoblotting with the corresponding antibodies23 (top panel). An analogous experiment was performed with MCM4-5FLAG (Control) and MCM4-5FLAG sld5-GA (the endogenous copy of SLD5 was modified to create sld5-GA, such that amino acids 5-9 were changed from Ile-Asp-Asp-Ile-Leu to Gly-Ala-Gly-Ala-Gly) (bottom panel).
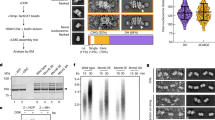
Extended Data Figure 7 2D EM analysis of Ctf4(CTD)–GINS complexes.
Comparison between GINS–Ctf4(CTD) complexes prepared by glycerol gradient or GraFix. a, SDS–PAGE gel of non-crosslinked GINS–Ctf4(CTD) complex. A red box highlights the fraction imaged by EM. b, Representative micrograph for the non-crosslinked preparation highlights small, globular particles. c, SDS–PAGE gel of crosslinked GINS–Ctf4(CTD) complex. A red box highlights the fraction imaged by EM. d, Representative micrograph for the crosslinked preparation highlights elongated features compatible with one, two or three GINS docked onto a Ctf4 trimerization core. e, Zoomed-in view of the same micrograph. f, Representative class averages of the Ctf4(CTD)–GINS complex show a mixture of complexes with clearly discernible stoichiometry: Ctf43–GINS, Ctf43–(GINS)2, Ctf43–(GINS)3. The box size is 448 Å.
Extended Data Figure 8 2D EM analysis of the Ctf4(CTD)–Pol1(NTD) complex.
a, Silver-stained SDS–PAGE gel showing the purified Ctf4(CTD)–Pol1(NTD) complex and GraFix gel of the same preparation. A red box highlights the fraction imaged by EM. b, Representative micrograph of the Ctf4(CTD)–Pol1(NTD) complex. c, Zoomed-in view of the same micrograph. d, Reference-free class averages of the Ctf4(CTD)–Pol1(NTD) complex highlight the presence of a ring-shaped core linked to one mobile globular density. The box size is 448 Å.
Extended Data Figure 9 2D EM analysis of Ctf4(CTD)–GINS–Pol1(NTD) complexes.
a, Silver-stained SDS–PAGE gel showing the purified GINS–Ctf4(CTD)–Pol1(NTD) complex and GraFix gel of the same preparation. A red box highlights the fraction imaged by EM. b, Representative micrograph of the GINS–Ctf4(CTD)–Pol1(NTD) complex. c, Zoomed-in view of the same micrograph. d, e, Reference-free class averages of the complex highlight the presence of a ring-shaped core linked to 2 or 3 peripheral features. The peripheral features fall into two categories: either elongated radially departing features similar to those seen for the Ctf4(CTD)–GINS complex, or smaller globular densities that were assigned to Pol1(NTD). The box size is 448 Å.
Supplementary information
Open and closed conformations of the Ctf4CTD trimer
The animation shows morphing between the open and closed forms observed in crystal structures of the Ctf4CTD trimer. The protein is drawn as ribbon, coloured according to its domain structure: the β-propeller domain is in light blue and the helical domain in yellow. (MOV 2470 kb)
Two-dimensional EM analysis of the Ctf4CTD - GINS interaction
The video shows a sequence of reference-free class averages of Ctf4CTD - GINS complexes, arranged in order of increasing GINS occupancy around the Ctf4CTD trimer. (MOV 9011 kb)
Rights and permissions
About this article
Cite this article
Simon, A., Zhou, J., Perera, R. et al. A Ctf4 trimer couples the CMG helicase to DNA polymerase α in the eukaryotic replisome. Nature 510, 293–297 (2014). https://doi.org/10.1038/nature13234
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13234
This article is cited by
-
Integrative bioinformatics analysis of WDHD1: a potential biomarker for pan-cancer prognosis, diagnosis, and immunotherapy
World Journal of Surgical Oncology (2023)
-
Chromatin-based DNA replication initiation regulation in eukaryotes
Genome Instability & Disease (2023)
-
Mechanisms of chromatin-based epigenetic inheritance
Science China Life Sciences (2022)
-
Chromatin replication and epigenetic cell memory
Nature Cell Biology (2020)
-
Characterization of the dimeric CMG/pre-initiation complex and its transition into DNA replication forks
Cellular and Molecular Life Sciences (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.