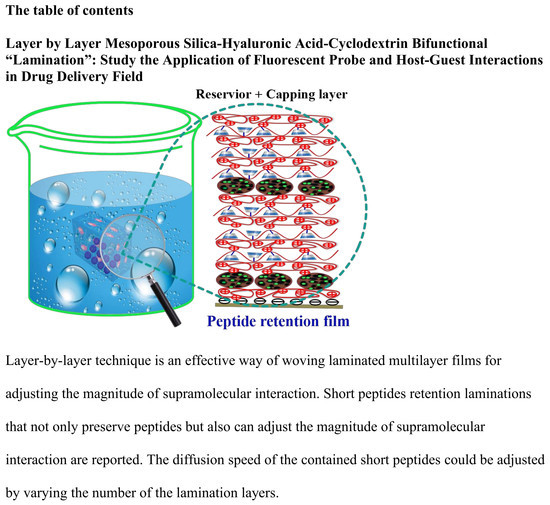
Layer by Layer Mesoporous Silica-Hyaluronic Acid-Cyclodextrin Bifunctional “Lamination”: Study of the Application of Fluorescent Probe and Host–Guest Interactions in the Drug Delivery Field
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. General UV-vis, Fluorescence Spectroscopy, Transmission Electron Microscopy (TEM) and Brunauer-Emmett-Teller (BET) Surface Area Analysis
2.3. The Synthesis of HA-CD Hydrogels
2.4. The Preparation of Mesoporous Silica Nanoparticles (MSN)
2.5. The Modification of Quartz Sheets
2.6. The Preparation of (PAH/SiO2)n/(PAH/HA–CD)n Layer-by-Layer Films
2.7. Adsorption and Sustained Release of Peptide
2.8. The Calculation Method of Diffusion Coefficient
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yao, M.S.; Lv, X.J.; Fu, Z.H.; Li, W.H.; Deng, W.H.; Wu, G.D.; Xu, G. Layer-by-Layer assembled conductive metal-organic framework nanofilms for room-temperature chemiresistive sensing. Angew. Chem. Int. Ed. 2017, 56, 16510–16514. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Meng, Y.H.; He, F.; Liu, E.Z.; Shi, C.S.; He, C.N.; Ma, L.Y.; Li, Q.Y.; Li, J.J.; Zhao, N.Q. Thermal decomposition-reduced layer-by-layer nitrogen-doped graphene/MoS2/nitrogen-doped graphene heterostructure for promising lithium-ion batteries. Nano Energy 2017, 41, 154–163. [Google Scholar] [CrossRef]
- Hoffmann, J.B.; Zaiats, G.; Wappes, I.; Kamat, P.V. CsPbBr3 Solar Cells: Controlled film growth through layer-by-layer quantum dot deposition. Chem. Mater. 2017, 29, 9767–9774. [Google Scholar] [CrossRef]
- Ribeiro, T.; Baleizao, C.; Farinha, J.P.S. Functional films from silica/polymer nanoparticles. Materials 2014, 7, 3881–3900. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.G.; Liu, Q.; Sanders, A.; Evans, J.S.; Smalyukh, I.I. Preparation of nanocomposite plasmonic films made from cellulose nanocrystals or mesoporous silica decorated with unidirectionally aligned gold nanorods. Materials 2014, 7, 3021–3033. [Google Scholar] [CrossRef] [PubMed]
- Eom, T.; Woo, K.; Cho, W.; Heo, J.E.; Jang, D.; Shin, J.I.; Martin, D.C.; Wie, J.J.; Shim, B.S. Nanoarchitecturing of natural melanin nanospheres by layer-by-layer assembly: Macroscale anti-inflammatory conductive coatings with optoelectronic tunability. Biomacromolecules 2017, 18, 1908–1917. [Google Scholar] [CrossRef] [PubMed]
- Andreou, I.; Amenitsch, H.; Likodimos, V.; Falaras, P.; Koutsoukos, P.G.; Leontidis, E. Organized silica films generated by evaporation-induced self-assembly as hosts for iron oxide nanoparticles. Materials 2013, 6, 1467–1484. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Nie, K.; Zhang, Y.H.; Wang, Y.; Hu, Y.M.; Dutschk, V.; Luan, X.L. PAH/DAS covalently cross-linked layer-by-layer multilayers: A “nano-net” superstratum immobilizes nanoparticles and remains permeable to small molecules. Soft Matter 2015, 11, 6859–6865. [Google Scholar] [CrossRef] [PubMed]
- Nie, K.; An, Q.; Zhang, Y.H. A functional protein retention and release multilayer with high stability. Nanoscale 2016, 8, 8791–8797. [Google Scholar] [CrossRef] [PubMed]
- Nie, K.; An, Q.; Tao, S.Y.; Zhang, Z.P.; Luan, X.L.; Zhang, Q.; Zhang, Y.H. Layer-by-layer reduced graphene oxide (rGO)/gold nanosheets (AuNSs) hybrid films: Significantly enhanced photothermal transition effect compared with rGO or AuNSs films. RSC Adv. 2015, 5, 57389–57394. [Google Scholar] [CrossRef]
- Ma, X.X.; Mei, L.F.; Liu, H.K.; Liao, L.B.; Nie, K.; Liu, Y.Q.; Li, Z.H. Synthesis and luminescence properties of Eu2+-activated phosphor Ba3LaK(PO4)3F for n-UV white-LEDs. Polyhedron 2016, 119, 223–226. [Google Scholar] [CrossRef]
- Ma, X.X.; Mei, L.F.; Liu, H.K.; Liao, L.B.; Liu, Y.Q.; Nie, K.; Li, Z.H. Structure and fluorescent properties of Ba3Sc(PO4)3:Sm3+ red-orange phosphor for n-UV w-LEDs. Chem. Phys. Lett. 2016, 653, 212–215. [Google Scholar] [CrossRef]
- Xiao, M.; Xian, Y.M.; Shi, F. Precise macroscopic supramolecular assembly by combining spontaneous locomotion driven by the marangoni effect and molecular recognition. Angew. Chem. Int. Ed. 2015, 54, 8952–8956. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.J.; Shi, F.; Li, J.S.; Lin, Z.F.; Jiang, C.; Xiao, M.; Zhang, L.Q.; Yang, W.T.; Nishi, T.S. Macroscopic supramolecular assembly of rigid building blocks through a flexible spacing coating. Adv. Mater. 2014, 26, 3009–3013. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.J.; Wang, Y.; Yu, L.L.; Su, H.J.; Han, W.D.; Lin, Z.F.; Li, J.S.; Hao, H.J.; Tong, C.; Li, X.L.; et al. Macroscopic supramolecular assembly to fabricate 3D ordered structures: Towards potential tissue scaffolds with targeted modification. Adv. Funct. Mater. 2015, 25, 6851–6857. [Google Scholar] [CrossRef]
- Yan, D.Y.; Zhou, Y.F.; Hou, J. Supramolecular self-assembly of macroscopic tubes. Science 2004, 303, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.J.; Zhang, Y.W.; Wang, S.; Shi, F. Macroscopic supramolecular assembly to fabricate multiplexed DNA patterns for potential application in DNA chips. Nanoscale 2017, 9, 17220–17223. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.J.; Ju, G.N.; Zhang, Y.W.; Song, M.M.; Zhang, Y.J.; Shi, F. Supramolecular assembly of macroscopic building blocks through self-propelled locomotion by dissipating chemical energy. Small 2014, 10, 3907–3911. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Wang, N.X.; Zhang, L.M.; Dai, L.R.; Shao, H.W.; Jiang, X.Y. Organic nanostructure-based probes for two-photon imaging of mitochondria and microbes with emission between 430 nm and 640 nm. Nanoscale 2017, 9, 4770–4776. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Sun, J.S.; Xian, Y.L.; Yin, B.F.; Niu, Y.J.; Wang, S.B.; Cao, F.J.; Zhang, X.Q.; Wang, Y.; Jiang, X.Y. A dual-readout chemiluminescent-gold lateral flow test for multiplex and ultrasensitive detection of disease biomarkers in real samples. Nanoscale 2016, 8, 15205–15212. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Feng, Q.; Sun, J.S.; Gao, F.; Fan, W.; Zhang, Z.; Li, X.H.; Jiang, X.Y. Nanocrystalline cellulose improves the biocompatibility and reduces the wear debris of ultrahigh molecular weight polyethylene via weak binding. ACS Nano 2016, 10, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, Y.; Dixon, P.; Sekar, P.; Chauhan, A. Incorporation of drug particles for extended release of cyclosporine a from poly-hydroxyethyl methacrylate hydrogels. Eur. J. Pharm. Biopharm. 2017, 120, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Paolino, M.; Ennen, F.; Lamponi, S.; Cernescu, M.; Voit, B.; Cappelli, A.; Appelhans, D.; Komber, H. Cyclodextrin-adamantane host–guest interactions on the surface of biocompatible adamantyl-modified glycodendrimers. Macromolecules 2013, 46, 3215–3227. [Google Scholar] [CrossRef]
- Guo, B.P.; Nie, H.L.; Yang, W.; Tian, Y.; Jing, J.; Zhang, X.L. A highly sensitive and rapidly responding fluorescent probe with a large Stokes shift for imaging intracellular hypochlorite. Sens. Actuators B-Chem. 2016, 236, 459–465. [Google Scholar] [CrossRef]
- Lee, C.H.; Cheng, S.H.; Huang, I.P.; Souris, J.S.; Yang, C.S.; Mou, C.Y.; Lo, L.W. Intracellular pH-responsive mesoporous silica nanoparticles for the controlled release of anticancer chemotherapeutics. Angew. Chem. Int. Ed. 2010, 49, 8214–8219. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Parida, S.; Maiti, C.; Mandal, M.; Dhara, D. pH-degradable and thermoresponsive water-soluble core cross-linked polymeric nanoparticles as potential drug delivery vehicle for doxorubicin. RSC Adv. 2015, 5, 83565–83575. [Google Scholar] [CrossRef]
- Narushima, K.; Hirata, S.; Vacha, M. Nanoscale triplet exciton diffusion via imaging of up-conversion emission from single hybrid nanoparticles in molecular crystals. Nanoscale 2017, 9, 10653–10661. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.M.; Kwac, K.; Li, M.; Jung, Y.; Park, H.G. Stability, molecular sieving, and ion diffusion selectivity of a lamellar membrane from two-dimensional molybdenum disulfide. Nano Lett. 2017, 17, 2342–2348. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Deng, W.Y.; Xiao, L.G.; Hu, Q.; Kan, Y.Y.; Chen, X.B.; Wang, C.; Huang, F.; Peng, J.B.; Wu, H.B.; et al. New insight of molecular interactions, crystallization and phase separation in higher performance small molecular solar cells via solvent vapor annealing. Nano Energy 2016, 30, 639–648. [Google Scholar] [CrossRef]
- Andrianov, A.K.; Marin, A.; Fuerst, T.R. Molecular-level interactions of polyphosphazene immunoadjuvants and their potential role in antigen presentation and cell stimulation. Biomacromolecules 2016, 17, 3732–3742. [Google Scholar] [CrossRef] [PubMed]
- Loren, N.; Hagman, J.; Jonasson, J.K.; Deschout, H.; Bernin, D.; Cella-Zanacchi, F.; Diaspro, A.; McNally, J.G.; Ameloot, M.; Smisdom, N.; et al. Fluorescence recovery after photobleaching in material and life sciences: Putting theory into practice. Q. Rev. Biophys. 2015, 48, 323–387. [Google Scholar] [CrossRef] [PubMed]
- Hagman, J.; Loren, N.; Hermansson, A.M. Probe diffusion in kappa-carrageenan gels determined by fluorescence recovery after photobleaching. Food Hydrocoll. 2012, 29, 106–115. [Google Scholar] [CrossRef]
- Meyvis, T.K.L.; De-Smedt, S.C.; Van-Oostveldt, P.; Demeester, J. Fluorescence recovery after photobleaching: A versatile tool for mobility and interactions measurements in pharmaceutical research. Pharm. Res. 1999, 16, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xiao, W.; Hu, C.; Xie, R.; Gao, H.L. Theranostic size-reducible and no donor conjugated gold nanocluster fabricated hyaluronic acid nanoparticle with optimal size for combinational treatment of breast cancer and lung metastasis. J. Control. Release 2018, 278, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Cun, X.L.; Ruan, S.B.; Liu, R.; Xiao, W.; Yang, X.T.; Yang, Y.Y.; Yang, C.Y.; Gao, H.L. Enzyme-triggered size shrink and laser-enhanced NO release nanoparticles for deep tumor penetration and combination therapy. Biomaterials 2018, 168, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yang, X.T.; Liu, R.; Ruan, S.B.; Zhou, Y.; Xiao, W.; Yu, W.Q.; Yang, C.Y.; Gao, H.L. Coadministration of iRGD with multistage responsive nanoparticles enhanced tumor targeting and penetration abilities for breast cancer therapy. ACS Appl. Mater. Interfaces 2018, 10, 22571–22579. [Google Scholar] [CrossRef] [PubMed]
- Chapeau, A.L.; Silva, J.V.C.; Schuck, P.; Thierry, A.; Floury, J. The influence of cheese composition and microstructure on the diffusion of macromolecules: A study using Fluorescence Recovery After Photobleaching (FRAP). Food Chem. 2016, 192, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Xia, H.X.; Yang, W.; Mou, P. New simulation-based approach for the profile control in a process chamber: Fluid, thermal, and plasma profile. Proc. Inst. Mech. Eng. E J. Process Mech. Eng. 2017, 231, 565–580. [Google Scholar] [CrossRef]
- Zhang, S.L.; Xu, T.Y.; Chai, S.C.; Zhang, L.Y.; Wu, L.X.; Li, H.L. Supramolecular star polymer films with tunable honeycomb structures templated by breath figures. Polymer 2017, 117, 306–314. [Google Scholar] [CrossRef]
- Iijima, K.; Aoki, D.; Otsuka, H.; Takata, T. Synthesis of rotaxane cross-linked polymers with supramolecular cross-linkers based on gamma-CD and PTHF macromonomers: The effect of the macromonomer structure on the polymer properties. Polymer 2017, 128, 392–396. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Garcia-Gonzalez, C.A.; Concheiro, A. Cyclodextrins as versatile building blocks for regenerative medicine. J. Control. Release 2017, 268, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Schibilla, F.; Voskuhl, J.; Fokina, N.A.; Dahl, J.E.P.; Schreiner, P.R.; Ravoo, B.J. Host–guest complexes of cyclodextrins and nanodiamonds as a strong non-covalent binding motif for self-assembled nanomaterials. Chem. Eur. J. 2017, 23, 16059–16065. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.G.; Malveau, C.; Mezour, M.A.; Perepichka, D.F.; Zhu, X.X. A molecular necklace: Threading beta-cyclodextrins onto polymers derived from bile acids. Angew. Chem. Int. Ed. 2016, 55, 11979–11983. [Google Scholar] [CrossRef] [PubMed]
- Tardy, B.L.; Tan, S.; Dam, H.H.; Ejima, H.; Blencowe, A.; Qiao, G.G.; Caruso, F. Nanoparticles assembled via pH-responsive reversible segregation of cyclodextrins in polyrotaxanes. Nanoscale 2016, 8, 15589–15596. [Google Scholar] [CrossRef] [PubMed]
- Trebosc, J.; Wiench, J.W.; Huh, S.; Lin, V.S.Y.; Pruski, M. Solid-state NMR study of MCM-41-type mesoporous silica nanoparticles. J. Am. Chem. Soc. 2005, 127, 3057–3068. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, D.; Koppel, D.E.; Schlessinger, J.; Elson, E.; Webb, W.W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys. J. 1976, 16, 1055–1069. [Google Scholar] [CrossRef] [Green Version]
- Soumpasis, D.M. Theoretical-analysis of fluorescence photobleaching recovery experiments. Biophys. J. 1983, 41, 95–97. [Google Scholar] [CrossRef]











© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, K.; An, Q.; Zink, J.I.; Yu, X.; Zhang, Y. Layer by Layer Mesoporous Silica-Hyaluronic Acid-Cyclodextrin Bifunctional “Lamination”: Study of the Application of Fluorescent Probe and Host–Guest Interactions in the Drug Delivery Field. Materials 2018, 11, 1745. https://doi.org/10.3390/ma11091745
Nie K, An Q, Zink JI, Yu X, Zhang Y. Layer by Layer Mesoporous Silica-Hyaluronic Acid-Cyclodextrin Bifunctional “Lamination”: Study of the Application of Fluorescent Probe and Host–Guest Interactions in the Drug Delivery Field. Materials. 2018; 11(9):1745. https://doi.org/10.3390/ma11091745
Chicago/Turabian StyleNie, Kun, Qi An, Jeffrey I. Zink, Xiang Yu, and Yihe Zhang. 2018. "Layer by Layer Mesoporous Silica-Hyaluronic Acid-Cyclodextrin Bifunctional “Lamination”: Study of the Application of Fluorescent Probe and Host–Guest Interactions in the Drug Delivery Field" Materials 11, no. 9: 1745. https://doi.org/10.3390/ma11091745