The Effect of Surface Charge on the Separation of Pyrite from Serpentine by Flotation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Methods
2.2.1. Micro-Flotation TESTS
2.2.2. Settlement Experiment
2.2.3. Microscopic Observation
2.2.4. Zeta Potential Measurements
2.2.5. FT-IR Spectroscopy
3. Results and Discussion
3.1. Effect of Mg2+ Ions on the Heterogeneous Flocculation Behavior of Pyrite and Serpentine
3.2. Dissolution and Phosphate Complexation of Mg2+ Ions on the Serpentine Surface
3.2.1. Effect of Mg2+ Ion Dissolution on the Zeta Potential of the Serpentine Surface
3.2.2. Phosphate Complexation on Surface Potentials of Serpentine
3.2.3. FT-IR Spectra of Phosphate Adsorption on the Serpentine Surface
3.3. Effect of Surface Charge on the Interaction Energy between Mineral Particles
3.4. Effect of Surface Potential Regulation on the Flotation Separation of Pyrite and Serpentine
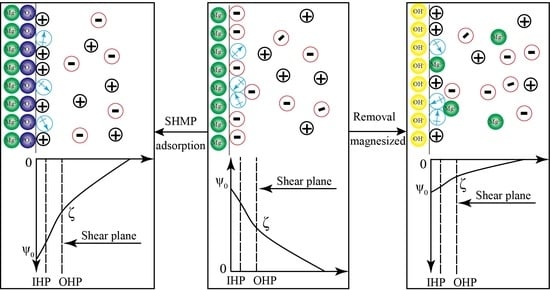
3.5. Mechanism Model of Surface Potential Regulation on the Serpentine Surface
4. Conclusions
- (1)
- Strengthened Mg2+ ion dissolution from the serpentine surface to the liquid phase can reduce the positive charge density of Mg2+ positioning ions in the double layer. This reduces the surface potential of serpentine, thereby eliminating heterogeneous condensation between serpentine and pyrite, and achieving good dispersion between mineral particles.
- (2)
- Chain-like polyphosphates can reduce the surface potential of serpentine. The mechanism mainly includes three aspects: They can enhance the dissolution of Mg2+ ions from the serpentine surface to the liquid phase and reduce the surface potential of serpentine; they can interact with Mg2+ in the liquid phase to form a stable soluble complex, thus maintaining the surface of serpentine; and the surface potential of serpentine is further reduced by adsorption onto the surface of serpentine.
- (3)
- Under natural conditions, the interfacial interaction between serpentine and pyrite in aqueous solution shows mutual attraction and heterogeneous coagulation between mineral particles. After adjusting the surface charge properties of serpentine, the interfacial interaction between serpentine and pyrite particles showed mutual exclusion, and mineral particles were dispersed in the solution system.
- (4)
- After adjusting the surface charge properties of serpentine, the dispersion of mineral particles was improved, and the artificial mixture of pyrite and serpentine could be effectively separated by flotation.
Author Contributions
Funding
Conflicts of Interest
References
- Rosenkranz, T.; Hipfinger, C.; Ridard, C.; Puschenreiter, M. A nickel phytomining field trial using Odontarrhena chalcidica and Noccaea goesingensis on an Austrian serpentine soil. J. Environ. Manag. 2019, 242, 522–528. [Google Scholar] [CrossRef]
- Yang, W.; Wang, G.; Wang, Q.; Dong, P.; Cao, H.; Zhang, K. Comprehensive recovery technology for Te, Au, and Ag from a telluride-type refractory gold mine. Minerals 2019, 9, 597. [Google Scholar] [CrossRef]
- Wu, S.; Ramandi, H.L.; Chen, H.; Crosky, A.; Hagan, P.; Saydam, S. Mineralogically influenced stress corrosion cracking of rockbolts and cable bolts in underground mines. Int. J. Rock Mech. Min. Sci. 2019, 119, 109–116. [Google Scholar] [CrossRef]
- Mobarak, M.; Mohamed, E.A.; Selim, A.Q.; Sellaoui, L.; Lamine, A.B.; Erto, A.; Bonilla-Petriciolet, A.; Seliem, M.K. Surfactant–modified serpentine for fluoride and Cr(VI) adsorption in single and binary systems: Experimental studies and theoretical modeling. Chem. Eng. J. 2019, 369, 333–343. [Google Scholar] [CrossRef]
- Li, Z.; Han, Y.; Li, Y.; Gao, P. Effect of serpentine and sodium hexametaphosphate on ascharite flotation. Trans. Nonferrous Met. Soc. China 2017, 27, 1841–1848. [Google Scholar] [CrossRef]
- Alvarez-Silva, M.; Uribe-Salas, A.; Waters, K.E.; Finch, J.A. Zeta potential study of pentlandite in the presence of serpentine and dissolved mineral species. Miner. Eng. 2016, 85, 66–71. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Y.; Zhang, X.; Qian, L.; Sun, M.; Yang, Y.; Zhang, Y.; Wang, J.; Kim, H.; Qiu, G. The dissolution and passivation mechanism of chalcopyrite in bioleaching: An overview. Miner. Eng. 2019, 136, 140–154. [Google Scholar] [CrossRef]
- Long, T.; Xiao, W.; Yang, W. The effect of molecular assembly between collectors and inhibitors on the flotation of pyrite and talc. R. Soc. Open Sci. 2019, 6, 191133. [Google Scholar] [CrossRef] [Green Version]
- Xiao, W.; Ren, Y.; Yang, J.; Cao, P.; Wang, J.; Qin, W.; Qiu, G. Adsorption mechanism of sodium oleate and styryl phosphonic acid on rutile and amphibole surfaces. Trans. Nonferrous Met. Soc. China 2019, 29, 1939–1947. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Q.; Wang, Y.; Dong, P. Eliminating the adverse effect of the lime on the gold-bearing pyrrhotite flotation using the isopentyl xanthate as collector at low alkalinity. Physicochem. Probl. Miner. Process. 2019, 55, 1250–1258. [Google Scholar]
- Zhang, C.; Liu, C.; Feng, Q.; Chen, Y. Utilization of N-carboxymethyl chitosan as selective depressants for serpentine on the flotation of pyrite. Int. J. Miner. Process. 2017, 163, 45–47. [Google Scholar] [CrossRef]
- Zhou, X.; Feng, B. The effect of polyether on the separation of pentlandite and serpentine. J. Mater. Res. Technol. 2015, 4, 429–433. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Yuan, Z.; Li, M.; Zhao, X.; Tong, Z.; Li, L.; Qi, S. The role of sodium oleate (NaOL) in the magnetic separation of pentlandite from serpentine using magnetic coating. Powder Technol. 2019, 345, 492–500. [Google Scholar] [CrossRef]
- Yang, S.; Xie, B.; Lu, Y.; Li, C. Role of magnesium-bearing silicates in the flotation of pyrite in the presence of serpentine slimes. Powder Technol. 2018, 332, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Lu, J.; Liu, J.; Li, L.; Wang, S. Enhancement of pentlandite surface magnetism and implications for its separation from serpentine via magnetic separation. Trans. Nonferrous Met. Soc. China 2017, 27, 204–210. [Google Scholar] [CrossRef]
- Wan, H.; Yang, W.; Cao, W.; He, T.; Liu, Y.; Yang, J.; Guo, L.; Peng, Y. The Interaction between Ca2+ and Molybdenite Edges and Its Effect on Molybdenum Flotation. Minerals 2017, 7, 141. [Google Scholar] [CrossRef]
- Ucbas, Y.; Bozkurt, V.; Bilir, K.; Ipek, H. Separation of chromite from serpentine in fine sizes using magnetic carrier. Sep. Sci. Technol. 2014, 49, 946–956. [Google Scholar] [CrossRef]
- Nie, X.; Feng, S.; Shudu, Z.; Quan, G. Simulation study on the dynamic ventilation control of single head roadway in high-altitude mine based on thermal comfort. Adv. Civ. Eng. 2019, 2019, 12. [Google Scholar] [CrossRef]
- Nie, X.; Wei, X.; Li, X.; Lu, C. Heat treatment and ventilation optimization in a deep mine. Adv. Civ. Eng. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Lu, J.; Sun, M.; Yuan, Z.; Qi, S.; Tong, Z.; Li, L.; Meng, Q. Innovative insight for sodium hexametaphosphate interaction with serpentine. Colloids Surf. A-Physicochem. Eng. Asp. 2019, 560, 35–41. [Google Scholar] [CrossRef]
- Zhao, K.; Yan, W.; Wang, X.; Gu, G.; Deng, J.; Zhou, X.; Hui, B. Dispersive effect of low molecular weight sodium polyacrylate on pyrite-serpentine flotation system. Physicochem. Probl. Miner. Process. 2017, 53, 1200–1213. [Google Scholar]
- Xiao, W.; Cao, P.; Liang, Q.; Huang, X.; Li, K.; Zhang, Y.; Qin, W.; Qiu, G.; Wang, J. Adsorption behavior and mechanism of Bi(III) ions on rutile-water interface in the presence of nonyl hydroxamic acid. Trans. Nonferrous Met. Soc. China 2018, 28, 348–355. [Google Scholar] [CrossRef]
- Xiao, W.; Cao, P.; Liang, Q.; Peng, H.; Zhao, H.; Qin, W.; Qiu, G.; Wang, J. The activation mechanism of Bi3+ ions to rutile flotation in a strong acidic environment. Minerals 2017, 7, 113. [Google Scholar] [CrossRef]
- Xiao, W.; Zhao, Y.; Yang, J.; Ren, Y.; Yang, W.; Huang, X.; Zhang, L. Effect of sodium oleate on the adsorption morphology and mechanism of nanobubbles on the mica surface. Langmuir 2019, 35, 9239–9245. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, X.; Zhou, L.; Zhou, W.; Wang, J.; Qin, W.; Qiu, G.; Hu, J.; Zhang, L. Influence of Mixing and Nanosolids on the Formation of Nanobubbles. J. Phys. Chem. B 2019, 123, 317–323. [Google Scholar] [CrossRef]
- He, T.; Li, H.; Jin, J.; Peng, Y.; Wang, Y.; Wan, H. Improving fine molybdenite flotation using a combination of aliphatic hydrocarbon oil and polycyclic aromatic hydrocarbon. Results Phys. 2019, 12, 1050–1055. [Google Scholar] [CrossRef]
- Li, H.; He, T.; Wang, Y.; Jin, J.; Yuan, H. XRD and SEM Analyses of Molybdenite with Different Particle Sizes and Its Floatability Difference. Spectrosc. Spectr. Anal. 2018, 38, 3588–3592. [Google Scholar]
- Huang, X.; Xiao, W.; Zhao, H.; Cao, P.; Hu, Q.; Qin, W.; Zhang, Y.; Qiu, G.; Wang, J. Hydrophobic flocculation flotation of rutile fines in presence of styryl phosphonic acid. Trans. Nonferrous Met. Soc. China 2018, 28, 1424–1432. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, T.; Duan, W.; Liang, S.; Li, G.; Xiao, W. Comparative studies on catalytic mechanisms for natural chalcopyrite-induced Fenton oxidation: Effect of chalcopyrite type. J. Hazard. Mater. 2020, 381, 120998. [Google Scholar] [CrossRef]
- Wang, S.; Xiao, W.; Ma, X.; Li, J.; Chen, L.; Yao, H.J.M. Analysis of the application potential of coffee oil as an ilmenite flotation collector. Minerals 2019, 9, 505. [Google Scholar] [CrossRef]
- Liu, Q.; Feng, X.; He, Y.; Lu, C.; Gu, Q. Three-dimensional multiple-relaxation-time lattice Boltzmann models for single-phase and solid-liquid phase-change heat transfer in porous media at the REV scale. Appl. Therm. Eng. 2019, 152, 319–337. [Google Scholar] [CrossRef]
- Bremmell, K.E.; Fornasiero, D.; Ralston, J. Pentlandite-lizardite interactions and implications for their separation by flotation. Colloids Surf. A Physicochem. Eng. Asp. 2005, 252, 207–212. [Google Scholar] [CrossRef]
- Feng, B.; Lu, Y.; Luo, X. The effect of quartz on the flotation of pyrite depressed by serpentine. J. Mater. Res. Technol. 2015, 4, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Elkin, A.P. Theoretical analysis of forces responsible for the interaction of lipid bilayer membranes in the contact zone. Biofizika 1977, 22, 444–447. [Google Scholar]
- Wang, J.; Gan, X.; Zhao, H.; Hu, M.; Li, K.; Qin, W.; Qiu, G. Dissolution and passivation mechanisms of chalcopyrite during bioleaching: DFT calculation, XPS and electrochemistry analysis. Miner. Eng. 2016, 98, 264–278. [Google Scholar] [CrossRef]









| Sample | MgO | SiO2 | Al2O3 | CaO | Fe | S | Other |
|---|---|---|---|---|---|---|---|
| Serpentine | 32.92 | 37.11 | 0.80 | 0.21 | 5.17 | - | - |
| Pyrite | - | - | - | - | 43.96 | 49.98 | 11.18 |
| Sample | Background Rate (%) | Number of Serpentine Particles |
|---|---|---|
| Pyrite + serpentine | 62.32 | 592 |
| Pyrite + demagnesium serpentine | 53.47 | 1188 |
| Pyrite + demagnesium serpentine + Mg2+ | 58.15 | 841 |
| Pyrite + serpentine + SHMP | 53.15 | 1147 |
| Mineral | Zeta Potential (mV) | ||
|---|---|---|---|
| Original | Demagnesium | SHMP | |
| Serpentine | 9.58 | −24.25 | −59.73 |
| Pyrite | −44.35 | −44.35 | −65.26 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, T.; Huang, X.; Xiao, W. The Effect of Surface Charge on the Separation of Pyrite from Serpentine by Flotation. Minerals 2019, 9, 629. https://doi.org/10.3390/min9100629
Long T, Huang X, Xiao W. The Effect of Surface Charge on the Separation of Pyrite from Serpentine by Flotation. Minerals. 2019; 9(10):629. https://doi.org/10.3390/min9100629
Chicago/Turabian StyleLong, Tao, Xiaotao Huang, and Wei Xiao. 2019. "The Effect of Surface Charge on the Separation of Pyrite from Serpentine by Flotation" Minerals 9, no. 10: 629. https://doi.org/10.3390/min9100629