High Quality Syngas Production with Supercritical Biomass Gasification Integrated with a Water–Gas Shift Reactor
Abstract
:1. Introduction
- Supercritical water has zero surface tension and most of the carbon-containing fuels are soluble in it, and therefore diffusion and penetration of water in the carbon with insignificant mass transfer resistance is plausible [23];
- The SCW gasification process is flexible with respect to the type of the carbonaceous fuel. For example, different types of biomass, coal, or even municipal waste with various contents of moisture and impurities are used as fuel sources [24];
- The required operating temperature for the gasification lies between 400 °C and 1000 °C depending on the type of the fuel and quality of the syngas;
- The produced carbon dioxide easily separates from H2 using pressurized water;
- Some physical properties of water, such as density, ion product, dielectric constant, viscosity, diffusivity, and solubility, change near or at its thermodynamic critical point (T = 374 °C and P = 22.1 MPa). At the critical point, water behaves similar to a dense gas with a consequent removal of any interphase mass transport processes. Organic compounds have high solubilities and complete miscibility with supercritical water [25];
- The process is high pressure, which reduces the costs related to the storage of the gaseous products such as post-compression operation.
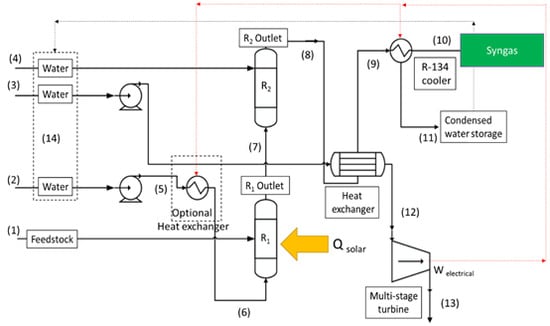
2. Methodology
- The reactions reach to the equilibrium;
- Heat loss is negligible from all reactors, pipes, tanks, and units;
- Heat and mass transfer coefficients are plausible to maintain the conditions for highest chemical performance of the reactions;
- Graphite in the present work is a surrogate for more realistic feedstock, which is used in the Gibbs minimization simulation. Any impurities in the feedstock have a negligible influence on the reactions and only carbon reacts in the supercritical gasifier. If there is an impurity, it is completely separated in the form of ash from the supercritical gasifier due to the difference between the ash and supercritical water density;
- The residence time is sufficient for the reactions to reach completion so that no unreacted carbon enters the WGS reactor;
- The process is isobar and the pressure of the reactors is the same.
3. Results and Discussion
3.1. Gibbs Free Energy and Enthalpy Assessment
3.2. Thermochemical Equilibrium Assessment for Reactor R1
3.3. Thermochemical Equilibrium Assessment for Reactor R2
3.4. Biomass Composition
4. Conclusions
- The addition of a water–gas shift reactor adds more control on the ratio of H2:CO and minimizes the production of CH4 and CO2. For the proposed system, the quality of syngas (H2:CO molar ratio) reaches 2.1 at P = 25 bar and 850 °C and 900 °C for reactors R1 and R2, respectively.
- Pressure was found to have no influence on the chemical performance of the water–gas shift reactor. However, pressurizing reactor R2 provides the pressure required for the storage of gas and reduces the cost of post-compression of products.
- The proposed system produces the syngas with different H2:CO ratios depending on the SCW/C and the temperature. For a given specific biomass, we found that syngas with the quality of 2.3 was produced at 900 °C and SCW/C = 2.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Turner, J.A. A realizable renewable energy future. Science 1999, 285, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Ogden, J.M.; Williams, R.H. Solar Hydrogen: Moving Beyond Fossil Fuels; World Resources Inst: Washington, DC, USA, 1989. [Google Scholar]
- Klass, D.L. Biomass for Renewable Energy, Fuels, and Chemicals; Academic Press: Cambridge, MA, USA, 1998. [Google Scholar]
- Dry, M.E. The fischer–tropsch process: 1950–2000. Catal. Today 2002, 71, 227–241. [Google Scholar] [CrossRef]
- Gemmen, R.; Trembly, J. On the mechanisms and behavior of coal syngas transport and reaction within the anode of a solid oxide fuel cell. J. Power Sources 2006, 161, 1084–1095. [Google Scholar] [CrossRef]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; Luis, F. Progress in chemical-looping combustion and reforming technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef]
- Jafarian, M.; Arjomandi, M.; Nathan, G.J. Thermodynamic potential of high temperature chemical looping combustion with molten iron oxide as the oxygen carrier. Chem. Eng. Res. Des. 2017, 120, 69–81. [Google Scholar] [CrossRef]
- Jafarian, M.; Arjomandi, M.; Nathan, G.J. A hybrid solar and chemical looping combustion system for solar thermal energy storage. Appl. Energy 2013, 103, 671–678. [Google Scholar] [CrossRef]
- Jafarian, M.; Arjomandi, M.; Nathan, G.J. A hybrid solar chemical looping combustion system with a high solar share. Appl. Energy 2014, 126, 69–77. [Google Scholar] [CrossRef]
- Jafarian, M.; Arjomandi, M.; Nathan, G.J. The energetic performance of a novel hybrid solar thermal & chemical looping combustion plant. Appl. Energy 2014, 132, 74–85. [Google Scholar]
- Tanner, J.; Bhattacharya, S. Kinetics of CO2 and steam gasification of Victorian brown coal chars. Chem. Eng. J. 2016, 285, 331–340. [Google Scholar] [CrossRef]
- Patra, T.K.; Sheth, P.N. Biomass gasification models for downdraft gasifier: A state-of-the-art review. Renew. Sustain. Energy Rev. 2015, 50, 583–593. [Google Scholar] [CrossRef]
- Chan, F.L.; Tanksale, A. Review of recent developments in Ni-based catalysts for biomass gasification. Renew. Sustain. Energy Rev. 2014, 38, 428–438. [Google Scholar] [CrossRef]
- Ge, H.; Guo, W.; Shen, L.; Song, T.; Xiao, J. Biomass gasification using chemical looping in a 25kW th reactor with natural hematite as oxygen carrier. Chem. Eng. J. 2016, 286, 174–183. [Google Scholar] [CrossRef]
- Adánez, J.; Gayán, P.; Celaya, J.; de Diego, L.F.; García-Labiano, F.; Abad, A. Chemical looping combustion in a 10 kWth prototype using a CuO/Al2O3 oxygen carrier: Effect of operating conditions on methane combustion. Ind. Eng. Chem. Res. 2006, 45, 6075–6080. [Google Scholar] [CrossRef]
- Li, F.; Kim, H.R.; Sridhar, D.; Wang, F.; Zeng, L.; Chen, J.; Fan, L.-S. Syngas chemical looping gasification process: Oxygen carrier particle selection and performance. Energy Fuels 2009, 23, 4182–4189. [Google Scholar] [CrossRef]
- Liao, C.; Wu, C.; Yan, Y. The characteristics of inorganic elements in ashes from a 1 MW CFB biomass gasification power generation plant. Fuel Process. Technol. 2007, 88, 149–156. [Google Scholar] [CrossRef]
- Fan, L.; Li, F.; Ramkumar, S. Utilization of chemical looping strategy in coal gasification processes. Particuology 2008, 6, 131–142. [Google Scholar] [CrossRef]
- Acharya, B.; Dutta, A.; Basu, P. Chemical-looping gasification of biomass for hydrogen-enriched gas production with in-process carbon dioxide capture. Energy Fuels 2009, 23, 5077–5083. [Google Scholar] [CrossRef]
- Anheden, M.; Svedberg, G. Exergy analysis of chemical-looping combustion systems. Energy Convers. Manag. 1998, 39, 1967–1980. [Google Scholar] [CrossRef]
- Zevenhoven-Onderwater, M.; Backman, R.; Skrifvars, B.-J.; Hupa, M. The ash chemistry in fluidised bed gasification of biomass fuels. Part I: Predicting the chemistry of melting ashes and ash–bed material interaction. Fuel 2001, 80, 1489–1502. [Google Scholar] [CrossRef]
- Florin, N. Calcium looping technologies for gasification and reforming. In Calcium and Chemical Looping Technology for Power Generation and Carbon Dioxide (CO2) Capture; Woodhead Publishing: Sawston, UK, 2015. [Google Scholar]
- Matsumura, Y.; Minowa, T.; Potic, B.; Kersten, S.R.A.; Prins, W.; van Swaaij, W.P.M.; van de Beld, B.; Elliott, D.C.; Neuenschwander, G.G.; Kruse, A. Biomass gasification in near-and super-critical water: Status and prospects. Biomass Bioenergy 2005, 29, 269–292. [Google Scholar] [CrossRef]
- Kruse, A. Supercritical water gasification. Biofuels Bioprod. Biorefining Innov. Sustain. Econ. 2008, 2, 415–437. [Google Scholar] [CrossRef]
- Williams, P.T.; Onwudili, J. Subcritical and supercritical water gasification of cellulose, starch, glucose, and biomass waste. Energy Fuels 2006, 20, 1259–1265. [Google Scholar] [CrossRef]
- Withag, J.A.; Smeets, J.R.; Bramer, E.A.; Brem, G. System model for gasification of biomass model compounds in supercritical water–a thermodynamic analysis. J. Supercrit. Fluids 2012, 61, 157–166. [Google Scholar] [CrossRef]
- Guan, Q.; Savage, P.E.; Wei, C. Gasification of alga Nannochloropsis sp. in supercritical water. J. Supercrit. Fluids 2012, 61, 139–145. [Google Scholar] [CrossRef]
- Yakaboylu, O.; Albrecht, I.; Harinck, J.; Smit, K.; Tsalidis, G.-A.; Di Marcello, M.; Anastasakis, K.; de Jong, W. Supercritical water gasification of biomass in fluidized bed: First results and experiences obtained from TU Delft/Gensos semi-pilot scale setup. Biomass Bioenergy 2018, 111, 330–342. [Google Scholar] [CrossRef]
- Guo, L.; Lu, Y.; Zhang, X.; Ji, C.; Guan, Y.; Pei, A. Hydrogen production by biomass gasification in supercritical water: A systematic experimental and analytical study. Catal. Today 2007, 129, 275–286. [Google Scholar] [CrossRef]
- Yu, D.; Aihara, M.; Antal, M.J., Jr. Hydrogen production by steam reforming glucose in supercritical water. Energy Fuels 1993, 7, 574–577. [Google Scholar] [CrossRef]
- Yong, T.L.-K.; Matsumura, Y. Reaction kinetics of the lignin conversion in supercritical water. Ind. Eng. Chem. Res. 2012, 51, 11975–11988. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, R.; Jin, H.; Lian, X.; Guo, L.; Huang, J. Supercritical water gasification of glycerol and glucose in different reactors: The effect of metal wall. Int. J. Hydrog. Energy 2016, 41, 16002–16008. [Google Scholar] [CrossRef]
- Reddy, S.N.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Supercritical water gasification of biomass for hydrogen production. Int. J. Hydrogen Energy 2014, 39, 6912–6926. [Google Scholar] [CrossRef]
- Tang, H.; Kitagawa, K. Supercritical water gasification of biomass: Thermodynamic analysis with direct Gibbs free energy minimization. Chem. Eng. J. 2005, 106, 261–267. [Google Scholar] [CrossRef]
- Castello, D.; Fiori, L. Supercritical water gasification of biomass: Thermodynamic constraints. Bioresour. Technol. 2011, 102, 7574–7582. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhao, L.; Han, Q.; Wei, L.; Zhang, X.; Guo, L.; Wei, J. Minimum fluidization velocities for supercritical water fluidized bed within the range of 633–693 K and 23–27 MPa. Int. J. Multiph. Flow 2013, 49, 78–82. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Jafarian, M.; Arjomandi, M.; Nathan, G.J. The relative performance of alternative oxygen carriers for liquid chemical looping combustion and gasification. Int. J. Hydrogen Energy 2017, 42, 16396–16407. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Jafarian, M.; Arjomandi, M.; Nathan, G.J. Potential of molten lead oxide for liquid chemical looping gasification (LCLG): A thermochemical analysis. Int. J. Hydrogen Energy 2018, 43, 4195–4210. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Jafarian, M.; Arjomandi, M.; Nathan, G.J. The thermo-chemical potential liquid chemical looping gasification with bismuth oxide. Int. J. Hydrogen Energy 2019, 44, 8038–8050. [Google Scholar] [CrossRef]
- Salari, E.; Peyghambarzadeh, M.; Sarafraz, M.M.; Hormozi, F. Boiling heat transfer of alumina nano-fluids: Role of nanoparticle deposition on the boiling heat transfer coefficient. Period. Polytech. Chem. Eng. 2016, 60, 252–258. [Google Scholar] [CrossRef]
- Salari, E.; Peyghambarzadeh, S.M.; Sarafraz, M.M.; Hormozi, F.; Nikkhah, V. Thermal behavior of aqueous iron oxide nano-fluid as a coolant on a flat disc heater under the pool boiling condition. Heat Mass Transf. 2017, 53, 265–275. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Arya, A.; Nikkhah, V.; Hormozi, F. Thermal performance and viscosity of biologically produced silver/coconut oil nanofluids. Chem. Biochem. Eng. Q. 2017, 30, 489–500. [Google Scholar] [CrossRef]
- Arya, A.; Sarafraz, M.M.; Shahmiri, S.; Madani, S.A.H.; Nikkhah, V.; Nakhjavani, S.M. Thermal performance analysis of a flat heat pipe working with carbon nanotube-water nanofluid for cooling of a high heat flux heater. Heat Mass Transf. 2018, 54, 985–997. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Arjomandi, M. Demonstration of plausible application of gallium nano-suspension in microchannel solar thermal receiver: Experimental assessment of thermo-hydraulic performance of microchannel. Int. Commun. Heat Mass Transf. 2018, 94, 39–46. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, S.Z.; Xu, D.H.; Gong, Y.M.; Ma, H.H.; Tang, X.Y. Review of catalytic supercritical water gasification for hydrogen production from biomass. Renew. Sustain. Energy Rev. 2010, 14, 334–343. [Google Scholar] [CrossRef]
- Chakinala, A.G.; Brilman, D.W.F.; van Swaaij, W.P.M.; Kersten, S.R.A. Catalytic and non-catalytic supercritical water gasification of microalgae and glycerol. Ind. Eng. Chem. Res. 2009, 49, 1113–1122. [Google Scholar] [CrossRef]
- Yanik, J.; Ebale, S.; Kruse, A.; Saglam, M.; Yüksel, M. Biomass gasification in supercritical water: II. Effect of catalyst. Int. J. Hydrogen Energy 2008, 33, 4520–4526. [Google Scholar] [CrossRef]
- Aziz, M. Integrated supercritical water gasification and a combined cycle for microalgal utilization. Energy Convers. Manag. 2015, 91, 140–148. [Google Scholar] [CrossRef]
- Wan, W. An innovative system by integrating the gasification unit with the supercritical water unit to produce clean syngas: Effects of operating parameters. Int. J. Hydrogen Energy 2016, 41, 14573–14582. [Google Scholar] [CrossRef]
- Wan, W. An innovative system by integrating the gasification unit with the supercritical water unit to produce clean syngas for solid oxide fuel cell (SOFC): System performance assessment. Int. J. Hydrogen Energy 2016, 41, 22698–22710. [Google Scholar] [CrossRef]
- Li, N.; Li, Y.; Ban, Y.; Song, Y.; Zhi, K.; Teng, Y.; He, R.; Zhou, H.; Liu, Q.; Qi, Y. Direct production of high hydrogen syngas by steam gasification of Shengli lignite/chars: Remarkable promotion effect of inherent minerals and pyrolysis temperature. Int. J. Hydrogen Energy 2017, 42, 5865–5872. [Google Scholar] [CrossRef]
- Calzavara, Y.; Joussot-Dubien, C.; Boissonnet, G.; Sarrade, S. Evaluation of biomass gasification in supercritical water process for hydrogen production. Energy Convers. Manag. 2005, 46, 615–631. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Indrawan, N.; Thapa, S.; Bhoi, P.R.; Huhnke, R.L.; Kumar, A. Engine power generation and emission performance of syngas generated from low-density biomass. Energy Convers. Manag. 2017, 148, 593–603. [Google Scholar] [CrossRef]
- Ong, Z.; Cheng, Y.; Maneerung, T.; Yao, Z.; Tong, Y.W.; Wang, C.H.; Dai, Y. Co-gasification of woody biomass and sewage sludge in a fixed-bed downdraft gasifier. AIChE J. 2015, 61, 2508–2521. [Google Scholar] [CrossRef]
- Zhai, Y.; Peng, C.; Xu, B.; Wang, T.; Li, C.; Zeng, G.; Zhu, Y. Hydrothermal carbonisation of sewage sludge for char production with different waste biomass: Effects of reaction temperature and energy recycling. Energy 2017, 127, 167–174. [Google Scholar] [CrossRef]
- Bustamante, F.; Enick, R.; Rothenberger, K.; Howard, B.; Cugini, A.; Ciocco, M.; Morreale, B. Kinetic study of the reverse water gas shift reaction in high-temperature, high pressure homogeneous systems. Fuel Chem. Div. Prepr. 2002, 47, 663–664. [Google Scholar]











| No. | Name | Reaction |
|---|---|---|
| 1 | Reforming | C + H2O(g)  H2(g) + CO(g) H2(g) + CO(g) |
| 2 | Partial oxidation of graphite | 3C + 2H2O(g)  CH4(g) + 2CO(g) CH4(g) + 2CO(g) |
| 3 | Complete oxidation of graphite | C + 2H2O(g)  2H2(g) + CO2(g) 2H2(g) + CO2(g) |
| 4 | Methanation | C + 2H2(g)  CH4(g) CH4(g) |
| 5 | Water-gas shift reaction | CO(g) + H2O(g)  H2(g) + CO2(g) H2(g) + CO2(g) |
| 6 | Boudouard reaction | CO2(g) + C  2CO(g) 2CO(g) |
| Parameter | T R1 (°C) | T R2 (°C) | Pressure (bar) | SCW/C |
|---|---|---|---|---|
| Range (min–max) | 650–1000 | 500–800 | 25–60 | 0.01–3 |
| Reference case | 650 | 600 | 25 | 1 |
| Feedstock | Proximate Analysis | Ultimate Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Biomass | Moisture Content | Ash | Volatile Matter | Fixed Carbon | C | H | N | S | O |
| Feedstock 1 | 55.31 | 60.32 | 30.32 | 9.36 | 22.3 | 3.3 | 2.1 | 0.5 | 11.3 |
| Feedstock 2 | 47.98 | 40.24 | 34.55 | 25.21 | 50.2 | 3.8 | 2.7 | 0.5 | 2.4 |
| Feedstock 3 | 41.39 | 52.67 | 32.19 | 15.14 | 37.8 | 3.1 | 2.3 | 0.42 | 3.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarafraz, M.M.; Safaei, M.R.; Jafarian, M.; Goodarzi, M.; Arjomandi, M. High Quality Syngas Production with Supercritical Biomass Gasification Integrated with a Water–Gas Shift Reactor. Energies 2019, 12, 2591. https://doi.org/10.3390/en12132591
Sarafraz MM, Safaei MR, Jafarian M, Goodarzi M, Arjomandi M. High Quality Syngas Production with Supercritical Biomass Gasification Integrated with a Water–Gas Shift Reactor. Energies. 2019; 12(13):2591. https://doi.org/10.3390/en12132591
Chicago/Turabian StyleSarafraz, M. M., Mohammad Reza Safaei, M. Jafarian, Marjan Goodarzi, and M. Arjomandi. 2019. "High Quality Syngas Production with Supercritical Biomass Gasification Integrated with a Water–Gas Shift Reactor" Energies 12, no. 13: 2591. https://doi.org/10.3390/en12132591