Boehmite Nanofillers in Epoxy Oligosiloxane Resins: Influencing the Curing Process by Complex Physical and Chemical Interactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Cycloaliphatic Epoxy Oligosiloxane (CEOS)
2.3. Nanocomposite Preparation and Film Curing
2.4. Characterization
3. Results and Discussion
3.1. Characterization of Cycloaliphatic Epoxy Oligosiloxane (CEOS)
3.2. Thermal Analysis of Cured CEOS and CEOS/BA Nanocomposites
3.3. Boehmite Distribution
3.4. UV Curing Kinetics
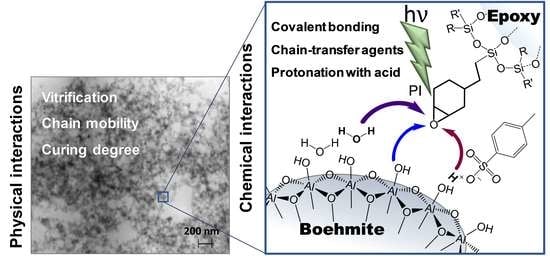
3.5. Proposed Curing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Müller, K.; Bugnicourt, E.; Latorre, M.; Jorda, M.; Echegoyen Sanz, Y.; Lagaron, J.M.; Miesbauer, O.; Bianchin, A.; Hankin, S.; Bölz, U.; et al. Review on the Processing and Properties of Polymer Nanocomposites and Nanocoatings and Their Applications in the Packaging, Automotive and Solar Energy Fields. Nanomaterials 2017, 7, 74. [Google Scholar] [CrossRef]
- Silvestre, J.; Silvestre, N.; De Brito, J. Polymer nanocomposites for structural applications: Recent trends and new perspectives. Mech. Adv. Mater. Struct. 2016, 23, 1263–1277. [Google Scholar] [CrossRef]
- Esposito, C.C.; Annalisa, C.; Mariaenrica, F. Measurements of size distribution nanoparticles in ultraviolet-curable methacrylate-based boehmite nanocomposites. J. Appl. Polym. Sci. 2013, 128, 4102–4109. [Google Scholar] [CrossRef]
- Hull, T.R.; Witkowski, A.; Hollingbery, L. Fire retardant action of mineral fillers. Degrad. Stab. 2011, 96, 1462–1469. [Google Scholar] [CrossRef] [Green Version]
- Sangermano, M.; Deorsola, F.A.; Fabiani, D.; Montanari, G.; Rizza, G. Epoxy-boehmite nanocomposites as new insulating materials. J. Appl. Sci. 2009, 114, 2541–2546. [Google Scholar] [CrossRef]
- Ghasem Zadeh Khorasani, M.; Silbernagl, D.; Szymoniak, P.; Hodoroaba, V.-D.; Sturm, H. The effect of boehmite (AlOOH) on nanomechanical and thermomechanical properties correlated to crosslinking density of epoxy in epoxy/boehmite nanocomposites. Polymer 2019, 164, 174–182. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Lendvai, L. Polymer/boehmite nanocomposites: A review. J. Appl. Polym. Sci. 2018, 135, 45573. [Google Scholar] [CrossRef]
- Shefer, K.I.; Cherepanova, S.V.; Moroz, É.M.; Gerasimov, E.Y.; Tsybulya, S.V. Features of the real structure of pseudoboehmites: Violations of the structure and layer packing caused by crystallization water. J. Struct. Chem. 2010, 51, 132–141. [Google Scholar] [CrossRef]
- Serra, A.; Ramis, X.; Fernández-Francos, X. Epoxy Sol-Gel Hybrid Thermosets. Coatings 2016, 6, 8. [Google Scholar] [CrossRef]
- Lebeau, B.; Sanchez, C. Sol-gel derived hybrid inorganic-organic nanocomposites for optics. Curr. Opin. Solid State Mater. Sci. 1999, 4, 11–23. [Google Scholar] [CrossRef]
- Decker, C.; Moussa, K. Kinetic investigation of photopolymerizations induced by laser beams. Macromol. Chem. 1990, 191, 963–979. [Google Scholar] [CrossRef]
- Decker, C.; Viet, T.N.T.; Thi, H.P. Photoinitiated cationic polymerization of epoxides. Polym. Int. 2001, 50, 986–997. [Google Scholar] [CrossRef]
- Golaz, B.; Michaud, V.; Leterrier, Y.; Månson, J.-A. UV intensity, temperature and dark-curing effects in cationic photo-polymerization of a cycloaliphatic epoxy resin. Polymer 2012, 53, 2038–2048. [Google Scholar] [CrossRef]
- Sangermano, M.; Razza, N.; Crivello, J.V. Cationic UV-Curing: Technology and Applications. Macromol. Mater. Eng. 2014, 299, 775–793. [Google Scholar] [CrossRef]
- Zhu, Q.; Liang, L.; Du, X.; Xiao, F.; Guo, Y.; Shi, J.; Wu, K.; Lu, M. Fabrication of High-Performance Cationic UV Curable Cycloaliphatic Epoxy/Silicone Hybrid Coatings. Macromol. Mater. Eng. 2018, 303, 1800020. [Google Scholar] [CrossRef]
- Teramoto, N.; Kogure, H.; Kimura, Y.; Shibata, M. Thermal properties and biodegradability of the copolymers of l-lactide, ε-caprolactone, and ethylene glycol oligomer with maleate units and their crosslinked products. Polymer 2004, 45, 7927–7933. [Google Scholar] [CrossRef]
- Ho, J.K.; Byeong-Soo, B. Synthesis and characterization of photopatternable epoxy hybrid materials for the fabrication of thick and thermally stable microstructures with a high aspect ratio. J. Appl. Polym. Sci. 2008, 108, 3169–3176. [Google Scholar] [CrossRef]
- Jin, J.; Lee, J.J.; Bae, B.-S.; Park, S.J.; Yoo, S.; Jung, K. Silica nanoparticle-embedded sol–gel organic/inorganic hybrid nanocomposite for transparent OLED encapsulation. Org. Electron. 2012, 13, 53–57. [Google Scholar] [CrossRef]
- Topolniak, I.; Chapel, A.; Gaume, J.; Bussiere, P.-O.; Chadeyron, G.; Gardette, J.-L.; Therias, S.; Chadeyron, G. Applications of polymer nanocomposites as encapsulants for solar cells and LEDs: Impact of photodegradation on barrier and optical properties. Degrad. Stab. 2017, 145, 52–59. [Google Scholar] [CrossRef]
- Xalter, R.; Halbach, T.S.; Mülhaupt, R. New Polyolefin Nanocomposites and Catalyst Supports Based on Organophilic Boehmites. Macromol. Symp. 2006, 236, 145–150. [Google Scholar] [CrossRef]
- www.products.sasol.com. Available online: https://products.sasol.com/pic/products/home/grades/ZA/5disperal-and-dispal/index.html (accessed on 9 May 2019).
- Hodoroaba, V.-D.; Rades, S.; Unger, W.E.S. Inspection of morphology and elemental imaging of single nanoparticles by high-resolution SEM/EDX in transmission mode. Surf. Interface Anal. 2014, 46, 945–948. [Google Scholar] [CrossRef]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Jung, K.; Bae, J.-Y.; Park, S.J.; Yoo, S.; Bae, B.-S. High performance organic-inorganic hybrid barrier coating for encapsulation of OLEDs. J. Mater. Chem. 2011, 21, 1977–1983. [Google Scholar] [CrossRef]
- Tipson, R.S. Infrared Absorption Spectra of p-Toluenesulfonic Acid and of Some of Its Esters. J. Am. Chem. Soc. 1952, 74, 1354. [Google Scholar] [CrossRef]
- Goertzen, W.; Kessler, M.; Kessler, M. Thermal and mechanical evaluation of cyanate ester composites with low-temperature processability. Compos. Part A Appl. Sci. Manuf. 2007, 38, 779–784. [Google Scholar] [CrossRef]
- Mutlur, S. Thermal Analysis of Composites Using DSC. In Advanced Topics in Characterization of Composites; Kessler, M.R., Ed.; Trafford Publishing: Bloomington, IN, USA, 2004; p. 202. [Google Scholar]
- Bokhimi, X.; Toledo-Antonio, J.; Guzmán-Castillo, M.; Hernández-Beltrán, F. Relationship between Crystallite Size and Bond Lengths in Boehmite. J. Solid State Chem. 2001, 159, 32–40. [Google Scholar] [CrossRef]
- Fankhänel, J.; Silbernagl, D.; Khorasani, M.G.Z.; Daum, B.; Kempe, A.; Sturm, H.; Rolfes, R. Mechanical Properties of Boehmite Evaluated by Atomic Force Microscopy Experiments and Molecular Dynamic Finite Element Simulations. J. Nanomater. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- Odian, G. Ionic Chain Polymerization. In Principles of Polymerization; Wiley: Hoboken, NJ, USA, 2004; pp. 372–463. [Google Scholar]
- Jabbour, J.; Calas, S.; Gatti, S.; Kribich, R.; Myara, M.; Pille, G.; Etienne, P.; Moreau, Y. Characterization by IR spectroscopy of an hybrid sol–gel material used for photonic devices fabrication. J. Non-Crystalline Solids 2008, 354, 651–658. [Google Scholar] [CrossRef]
- Gao, N.; Liu, W.; Yan, Z.; Wang, Z. Synthesis and properties of transparent cycloaliphatic epoxy–silicone resins for opto-electronic devices packaging. Opt. Mater. 2013, 35, 567–575. [Google Scholar] [CrossRef]
- Corcione, C.E.; Frigione, M.; Maffezzoli, A.; Malucelli, G. Photo – DSC and real time – FT-IR kinetic study of a UV curable epoxy resin containing o-Boehmites. Eur. J. 2008, 44, 2010–2023. [Google Scholar]
- Park, S.-J.; Heo, G.-Y.; Suh, D.-H. Thermal properties and fracture toughness of epoxy resins cured by phosphonium and pyrazinium salts as latent cationic initiators. J. Sci. Part A Chem. 2003, 41, 2393–2403. [Google Scholar] [CrossRef]
- Penczek, S.; Kubisa, P.; Szymanski, R. Activated monomer propagation in cationic polymerizations. Makromol. Chemie Macromol. Symp. 1986, 3, 203–220. [Google Scholar] [CrossRef]
- Li, Y.-S.; Li, M.-S.; Chang, F.-C.; Li, Y.; Chang, F. Kinetics and curing mechanism of epoxy and boron trifluoride monoethyl amine complex system. J. Sci. Part A Chem. 1999, 37, 3614–3624. [Google Scholar] [CrossRef]
- Tokar, R.; Kubisa, P.; Penczek, S.; Dworak, A. Cationic polymerization of glycidol: coexistence of the activated monomer and active chain end mechanism. Macromolecules 1994, 27, 320–322. [Google Scholar] [CrossRef]
- Akatsuka, M.; Takezawa, Y.; Amagi, S. Influences of inorganic fillers on curing reactions of epoxy resins initiated with a boron trifluoride amine complex. Polymer 2001, 42, 3003–3007. [Google Scholar] [CrossRef]
- Rajabi, L.; Marzban, M.; Derakhshan, A.A. Epoxy/alumoxane and epoxy/boehmite nanocomposites: Cure behavior, thermal stability, hardness and fracture surface morphology. Iran. J. 2014, 23, 203–215. [Google Scholar] [CrossRef]
- Kubisa, P.; Penczek, S. Cationic activated monomer polymerization of heterocyclic monomers. Prog. Sci. 1999, 24, 1409–1437. [Google Scholar] [CrossRef]
- Choi, G.-M.; Jin, J.; Shin, D.; Kim, Y.H.; Ko, J.-H.; Im, H.-G.; Jang, J.; Jang, D.; Bae, B.-S. Flexible Hard Coating: Glass-Like Wear Resistant, Yet Plastic-Like Compliant, Transparent Protective Coating for Foldable Displays. Adv. Mater. 2017, 29, 1700205. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, R.; Turcato, E.A.; Di Gianni, A.; Ronchetti, S. Epoxy coatings containing clays and organoclays: Effect of the filler and its water content on the UV-curing process. Prog. Org. Coat. 2008, 62, 336–343. [Google Scholar] [CrossRef]















| DISPERAL® HP14 | DISPERAL® OS1 | |
|---|---|---|
| Mean crystallite size (120) [nm] | 14 | 10 |
| Surface area 1 (m2/g) | 160 | 240 |
| Loose bulk density (g/cm3) | 400–600 | 400–600 |
| Surface treatment | - | p-toluenesulfonic acid |
| Tg (°C) | ΔCp (J/g/°C) | |
|---|---|---|
| CEOS | 30 ± 2 | 0.23 ± 0.01 |
| CEOS/HP14-1% | 52 ± 3 | 0.20 ± 0.02 |
| CEOS/HP14-2% | 54 ± 2 | 0.16 ± 0.04 |
| CEOS/HP14-5% | 48 ± 2 | 0.16 ± 0.02 |
| CEOS/HP14-10% | 45 ± 3 | 0.15 ± 0.02 |
| CEOS/OS1-1% | 64 ± 5 | 0.10 ± 0.06 |
| CEOS/OS1-2% | 73 ± 4 | 0.05 ± 0.05 |
| CEOS/OS1-5% | 71 ± 3 | 0.07 ± 0.02 |
| CEOS/OS1-10% | 65 ± 4 | 0.08 ± 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Topolniak, I.; Hodoroaba, V.-D.; Pfeifer, D.; Braun, U.; Sturm, H. Boehmite Nanofillers in Epoxy Oligosiloxane Resins: Influencing the Curing Process by Complex Physical and Chemical Interactions. Materials 2019, 12, 1513. https://doi.org/10.3390/ma12091513
Topolniak I, Hodoroaba V-D, Pfeifer D, Braun U, Sturm H. Boehmite Nanofillers in Epoxy Oligosiloxane Resins: Influencing the Curing Process by Complex Physical and Chemical Interactions. Materials. 2019; 12(9):1513. https://doi.org/10.3390/ma12091513
Chicago/Turabian StyleTopolniak, Ievgeniia, Vasile-Dan Hodoroaba, Dietmar Pfeifer, Ulrike Braun, and Heinz Sturm. 2019. "Boehmite Nanofillers in Epoxy Oligosiloxane Resins: Influencing the Curing Process by Complex Physical and Chemical Interactions" Materials 12, no. 9: 1513. https://doi.org/10.3390/ma12091513