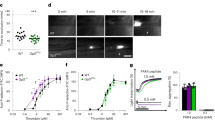
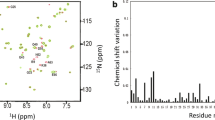
Abstract
AT sites of vascular injury, thrombin interacts with multiple procoagulant substrates1–6 to mediate both fibrin clotting and platelet aggregation. But upon binding to thrombomodulin on the vascular endothelium, thrombin instead activates protein C, thereby functioning as an anticoagulant and attenuating clot formation7. Upon infusion in vivo, both the procoagulant and anticoagulant effects of thrombin were observed8,9. Preliminary studies indicating that thrombin's protein C activating and fibrinogen clotting activities could be dissociated by mutagenesis10 suggested to us that a thrombin variant that lacked procoagulant activity while retaining anti-coagulant function might be an attractive antithrombotic agent. Using protein engineering, we introduced a single substitution, E229A, that substantially shifted thrombin's specificity in favour of the anticoagulant substrate, protein C. In monkeys, this modified thrombin functioned as an endogenous protein C activator demonstrating dose-dependent, reversible anticoagulation without any indication of procoagulant activity. Notably, template bleeding times were not prolonged, suggesting a reduced potential for bleeding complications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Jackson, C. M. & Nemerson, Y. A. Rev. Biochem. 49, 765–811 (1980).
Mann, K. G. & Lundblad, R. L. in Hemostasis and Thrombosis (eds Colman, R. W., Hirsh, J., Marder, V. J. & Salzman, E. W.) 148–161 (Lippincott, Philadelphia, PA, 1987).
Vu, T. K. H., Wheaton, V. I., Hung, D. T., Charo, I. & Coughlin, S. R. Nature 353, 674 (1991).
Lorand, L. & Radek, J. T. in Thrombin Structure and Function (ed. Berliner, L. J.) 257–270 (Plenum, Columbus, OH, 1992).
Gailani, D. & Broze, G. J. Jr Science 253, 909–912 (1991).
Mann, K. G., Jenny, R. J. & Krishnaswamy, S. A. Rev. Biochem. 57, 915–956 (1988).
Esmon, C. T. Science 235, 1348–1352 (1987).
Comp, P. C., Jacocks, R. M., Ferrell, G. L. & Esmon, C. T. J. clin. Invest. 70, 127–134 (1982).
Hanson, S. R. et al. J. clin. Invest. 92, 2003–2012 (1993).
Wu, Q. et al. Proc. natn. Acad. Sci. U.S.A. 88, 6675–6779 (1991).
Tsiang, M. et al. J. biol. Chem. 270, 16854–16863 (1995).
Stubbs, M. T. et al. Eur. J. Biochem. 206, 187–195 (1992).
Banfield, D. K. & MacGillivray, R. T. A. Proc. natn. Acad. Sci. U.S.A. 89, 2779–2783 (1992).
Ni, F., Konishi, Y., Bullock, L. D., Rivetna, M. N. & Scheraga, H. A. Biochemistry 28, 3106–3119 (1989).
Borowski, M., Furie, B. C., Goldsmith, G. H. & Furie, B. J. biol. Chem. 260, 9258–9264 (1985).
Fenton, J. W., Fasco, M. J. & Stackrow, A. B. J. biol. Chem. 252, 3587–3598 (1977).
Tsiang, M. et al. J. biol. Chem. 270, 19370–19376 (1995).
Higgins, D. L. J. biol. Chem. 258, 9276–9282 (1983).
Graycar, T. P. & Estell, D. A. J. cell. Biochem. 11c (suppl.), 234 (1987).
Tsiang, M., Lentz, S. R. & Sadler, J. E. J. biol. Chem. 267, 6164–6170 (1992).
Owen, W. G. & Esmon, C. T. J. biol. Chem. 256, 5532–5535 (1981).
Ye, J. et al. J. biol. Chem. 269, 17965–17970 (1994).
Dang, Q. D. et al. Proc. natn. Acad. Sci. U.S.A. 92, 5977–5981 (1995).
National Academy of Sciences Guide for Care and Use of Laboratory Animals 85–23 (National Institutes of Health, Washington DC, 1985).
Orthner, C. L., Kolen, B. & Drohan, W. N. Thromb. Haemostasis 69, 441–447 (1993).
Gruber, A. & Griffin, J. H. Blood 79, 2340–2348 (1992).
Gruber, A. et al. Circulation 84, 2454–2462 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gibbs, C., Coutré, S., Tsiang, M. et al. Conversion of thrombin into an anticoagulant by protein engineering. Nature 378, 413–416 (1995). https://doi.org/10.1038/378413a0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/378413a0
This article is cited by
-
rAAV-mediated over-expression of acid ceramidase prevents retinopathy in a mouse model of Farber lipogranulomatosis
Gene Therapy (2023)
-
Residues W215, E217 and E192 control the allosteric E*-E equilibrium of thrombin
Scientific Reports (2019)
-
Interplay between conformational selection and zymogen activation
Scientific Reports (2018)
-
Rational Design of Protein C Activators
Scientific Reports (2017)
-
Thrombin: A paradigm for enzymes allosterically activated by monovalent cations
Rendiconti Lincei (2006)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.