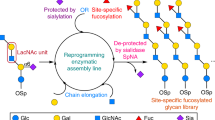
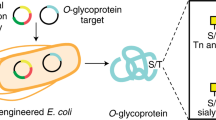
Abstract
Synthetic glycoconjugates that mimic cell-surface tumour antigens (glycolipids or glycoproteins with unusual carbohydrate structural motifs) have been shown to trigger humoral responses in murine and human immune systems1,2,3. This raises the exciting possibility of inducing active immunity with fully synthetic carbohydrate vaccines, particularly if vaccine compounds can be synthesized that resemble the surface environment of transformed cells even more closely. Glycopeptides seem particularly suitable for this purpose. In contrast to most glycolipids and thecarbohydrates themselves, glycopeptides bind to major histocompatibility complex molecules, and, in favourable cases, can stimulate T cells and lead to the expression of receptors that recognize the carbohydrate part of a glycopeptide with high specificity4,5,6,7,8. The preparation of glycopeptides and glycoproteins remains, however, a difficult challenge9,10,11,12: earlier synthesis methods have been inefficient, and established cloning approaches that allow engineering of global glycopatterns produce only heterogeneous glycoproteins13. Here we report an efficient strategy of the synthesis of tumour-associated mucin glycopeptides with clustered trisaccharide glycodomains corresponding to the (2,6)-sialyl T antigen. Our approach involves construction of the complete glycodomain in the first stage, followed by convergent coupling to amino acid residues and subsequent incorporation of the glycosyl amino acid units into a peptide chain. This general strategy allows the assembly of molecules in which selected glycoforms can be incorporated at any desired position of the peptide chain. The resultant fully synthetic O-linked glycopeptide clusters are the closest homogeneous mimics of cell-surface mucins at present available, and so are promising compounds for the development of anticancer vaccines.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ragupathi, G. et al. Immunization of mice with a fully synthetic globo-H antigen results in antibodies against human cancer cells: A combined chemical–immunological approach to the fashioning of an anticancer vaccine Angew. Chem. Int. Edn Engl. 36, 125–128 (1997).
Toyokuni, T. & Singhal, A. K. Synthetic carbohydrate vaccines based on tumor-associated antigens. Chem. Soc. Rev. 24, 231–242 (1995).
Bilodeau, M. T. & Danishefsky, S. J. Glycals in organic synthesis: the evolution of compreshensive strategies for the assembly of oligosaccharides and glycoconjugates of biological consequence. Angew. Chem. Int. Edn Engl. 35, 1381–1419 (1996).
Deck, B., Elofsson, K., Kihlberg, J. & Unanue, E. E. Specificity of glycopeptide-specific T cells. J. Immunol. 155, 1074–1078 (1995).
Haurum, J. S. et al. Recognition of carbohydrate by major histocompatibility complex class I-restricted, glycopeptide-specific cytotoxic T lymphocytes. J. Exp. Med. 180, 739–744 (1994).
Mouritsen, S. et al. Attachment of oligosaccharides to peptide antigen profoundly affects binding to major histocompatibility complex class II molecules and peptide immunogenicity. Eur. J. Immunol. 24, 1066–1072 (1994).
Sieling, P. A. et al. CD1-restricted T cell recognition of microbial lipoglycan antigens. Science 269, 227–230 (1995).
Jenson, T. et al. Carbohydrate and peptide specificity of MHC Class II-restricted T cell hybridomas raised against an O-glycosylated self peptide. J. Immunol. 3769–3778 (1997).
Bill, R. M. & Flitsch, S. L. Chemical and biological approaches to glycoprotein synthesis. Chem. Biol. 3, 145–149 (1996).
Witte, K., Sears, P., Martin, K. & Wong, C.-H. Enzymatic glycoprotein synthesis: preparation of ribonuclease glycoforms via enzymatic glycopeptide condensation and glycosylation. J. Am. Chem. Soc. 119, 2114–2118 (1997).
Tsuda, T. & Nithimura, S.-I. Synthesis of an antifreeze glycoprotein analogue: efficient preparation of sequential glycopeptide polymers. J. Chem. Soc. Chem. Commun. 2779–2780 (1996).
Nakahara, Y., Iijima, H. & Ogawa, T. in Synthetic Oligosaccharides, Indispensable Probes for the Life Sciences (ed. P. Kovác) 249–266 (ACS Symp. Ser. 560, ACS, Washington DC, (1994).
Jenkins, N., Parekh, R. B. & James, D. C. Getting the glycosylation right: Implications for the biotechnology industry. Nature Biotechnol. 14, 975–981 (1996).
Carlstedt, I. & Davies, J. R. Glycoconjugates facing the outside world. Biochem Soc. Trans. 25, 214–219 (1997).
Finn, O. J. et al. MUC-1 epithelial tumor mucin-based immunity and cancer vaccines. Immunol. Rev. 145, 61–89 (1995).
Fukuda, M., Carlsson, S. R., Klock, J. C. & Dell, A. Structures of O−linked oligosaccharides isolated from normal granulocytes, chronic myelogenous leukemia cells, and acute myelogenous leukemia cells. J. Biol. Chem. 261, 12796–12806 (1986).
Saitoh, O., Gallagher, R. E. & Fukuda, M. Expression of aberrant O−glycans attached to leukosialin in differentiation-deficient HL-60 cells. Cancer Res. 51, 2854–2862 (1991).
Pallant, A. et al. Characterization of cDNAs encoding human leukosialin and localization of the leukosialin gene to chromosome 16. Proc. Natl Acad. Sci. USA 86, 1328–1332 (1989).
Kunz, H. & Schultz, M. in Glycopeptides and Related Compounds (eds Large, D. G. & Warren, Ch. D.) 23 (Dekker, New York, (1997).
Paulsen, H. et al. New solid phase oligosaccharide synthesis on glycopeptides bound to a solid phase. J. Chem. Soc. Perkin Trans. 1 281–293 (1997).
Liebe, B. & Kunz, H. Solid phase synthesis of a tumor-associated sialyl-Tn antigen glycopeptide with a partial sequence of the “tandem repeat” of the Muc-1 mucin. Angew. Chem. Int. Edn Engl. 36, 618–621 (1997).
Qui, D. & Koganty, R. R. Mucin type glycopeptides: synthesis of core 2, core 6 and F1-α building blocks and some unexpected reactions. Tetrahedr. Lett. 38, 45–48 (1997).
Elofsson, M., Salvador, L. A. & Kihlberg, J. Preparation of Tn and sialyl Tn building blocks used in Fmoc solid-phase synthesis of glycopeptide fragments from HIV gp120. Tetrahedron 53, 369–390 (1997).
Szabo, L., Ramza, J., Langdon, C. & Polt, R. Stereoselective synthesis of O−serinyl/threonyl-2-acetamido-2-deoxy-α or β-glycosides. Carbohydr. Res. 274, 11–28 (1995).
Martin, T. J., Brescello, R., Toepfer, A. & Schmidt, R. R. Synthesis of phosphites and phosphates of neuraminic acid and their glycosyl donor properties — convenient synthesis of GM3. Glycoconjugate J. 10, 16–25 (1993).
Sim, M. M., Kondo, H. & Wong, C.-H. Synthesis and use of glycosyl phosphites: an effective route to glycosyl phosphates, sugar nucleotides and glycosides. J. Am. Chem. Soc. 115, 2260–2267 (1993).
Schmidt, R. R. & Kinzy, W. Anomeric oxygen activation for glycoside synthesis. Adv. Carbohydr. Chem. Biochem. 50, 84–123 (1994).
Kondo, H. et al. Glycosyl phosphites as glycosylation reagents: Scope and mechanism. J. Org. Chem. 59, 864–867 (1994).
Paulsen, H., Rauwald, W. & Weichert, U. Glycosidierung mit thioglycosiden von oligosacchariden zu segmenten von O-glycoproteinen. Liebigs Ann. Chem. 75–86 (1988).
Iijima, H. & Ogawa, T. Synthesis of a mucin type O -glycosylated amino acid, β-Gal (1 → 3)-[α-Neu5Ac-(2 → 6)]-α-GalNac-(1 → 3)-Ser. Carbohydr. Res. 186, 95–106 (1989).
Jiang, J., Li, W.-R. & Joullié, M. Selective removal of fluorenylmethoxycarbonyl (Fmoc) groups under mild conditions. Synth. Commun. 24, 187–195 (1994).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sames, D., Chen, XT. & Danishefsky, S. Convergent total synthesis of a tumour-associated mucin motif. Nature 389, 587–591 (1997). https://doi.org/10.1038/39292
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/39292
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.