Abstract
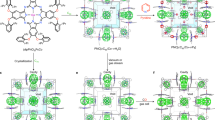
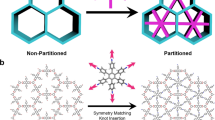
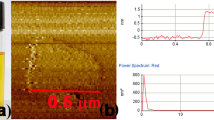
Microporous oxides are inorganic materials with wide applications in separations, ion exchange and catalysis1,2,3. In such materials, an important determinant of pore size is the number of M (where M = Si, Ge and so on) atoms in the rings delineating the channels1. The important faujasite structure exhibits 12-ring structures while those of zeolites4,5, germanates6,7,8 and other8 materials can be much larger. Recent attention has focused on mesoporous materials with larger pores of nanometre scale9,10,11; however, with the exception of an inorganic–organic hybrid12, these have amorphous pore walls, limiting many applications. Chiral porous oxides are particularly desirable for enantioselective sorption and catalysis13. However, they are very rare in microporous14,15 and mesoporous16 materials. Here we describe a mesoporous germanium oxide, SU-M, with gyroidal channels separated by crystalline walls that lie about the G (gyroid) minimal surface as in the mesoporous MCM-48 (ref. 9). It has the largest primitive cell and lowest framework density of any inorganic material and channels that are defined by 30-rings. One of the two gyroidal channel systems of SU-M can be filled with additional oxide, resulting in a mesoporous crystal (SU-MB) with chiral channels.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Davis, M. E. Ordered porous materials for emerging applications. Nature 417, 813–821 (2002)
Cheetham, A. K., Férey, G. & Loiseau, T. Open-framework inorganic materials. Angew. Chem. Int. Edn Engl. 38, 3269–3292 (1999)
van Bekkum, H., Jacobs, P. A., Flanigen, E. M. & Jansen, J. C. (eds) Introduction to Zeolite Science and Practice 2nd edn (Elsevier, New York, 2001)
Davis, M. E., Saldarriaga, C., Montes, C., Garces, C. & Crowder, C. A molecular sieve with 18-membered rings. Nature 331, 698–699 (1988)
Estermann, M., McCusker, L. B., Baerlocher, Ch., Merrouche, A. & Kessler, H. A synthetic gallophosphate molecular-sieve with a 20-tetrahedral-atom pore opening. Nature 352, 320–323 (1991)
Plévert, J. et al. A flexible germanate structure containing 24-ring channels and with a very low framework density. J. Am. Chem. Soc. 123, 12706–12707 (2001)
Zhou, Y. et al. A large 24-membered-ring germanate zeolite-type open-framework structure with three-dimensional intersecting channels. Angew. Chem. Int. Edn Engl. 40, 2166–2168 (2001)
Tang, L., Dadachov, M. S. & Zou, X. D. SU-12: a silicon-substituted ASU-16 with circular 24-rings and templated by a monamine. Chem. Mater. 17, 2530–2536 (2005)
Kresge, C. T., Leonowicz, M. E., Roth, W. J., Vartuli, J. C. & Beck, J. S. Ordered mesoporous molecular sieves synthesized by a liquid crystal template mechanism. Nature 359, 710–712 (1992)
Zhao, D. et al. Triblock copolymer synthesis of mesoporous silica with periodic 50 to 300 ångstrom pores. Science 279, 548–552 (1998)
Terasaki, O. (ed.) Mesoporous and Related Nano-structured Materials (Elsevier, New York, 2004)
Inagaki, S., Guan, S., Ohsuna, T. & Terasaki, O. An ordered mesoporous organosilica hybrid material with crystal-like wall structure. Nature 416, 304–307 (2002)
Baiker, A. Chiral catalysis on solids. Curr. Opin. Solid State Mater. Sci. 3, 86–93 (1998)
Gier, T. E., Bu, X., Feng, P. & Stucky, G. D. Synthesis and organization of zeolite- like materials with three-dimensional helical pores. Nature 395, 154–157 (1998)
Wang, Y., Yu, J., Guo, M. & Xu, R. [{Zn2(HPO4)4}{Co(dien)2}]H3O: A zinc phosphate with multidirectional intersecting helical channels. Angew. Chem. Int. Edn Engl. 42, 4089–4092 (2003)
Che, S. et al. Synthesis and characterization of chiral mesoporous silica. Nature 429, 281–284 (2004)
Andersson, S. & O'Keeffe, M. Body-centered cubic cylinder packing and the garnet structure. Nature 267, 605–606 (1976)
Müller, A., Koop, M., Bögge, H., Schmidtmann, M. & Beugholt, C. Exchanged ligands on the surface of a giant cluster: [(MoO3)176(H2O)63(CH3OH)17Hn](32-n)-. Chem. Commun. 1501–1502 (1998)
O'Keeffe, M., Yaghi, O. M., Moler, D., Joshi, G., Ockwig, N. & Delgado-Friedrichs, O. Reticular Chemistry Structure Resource http://okeeffe-ws1.la.asu.edu/RCSR/home.htm (Arizona State Univ., Arizona, 2004)
Delgado-Friedrichs, O., O'Keeffe, M. & Yaghi, O. M. Three-periodic nets and tilings: regular and quasiregular nets. Acta Crystallogr. A 59, 22–27 (2003)
Andersson, S., Hyde, S. T., Larsson, K. & Lidin, S. Minimal surfaces and structures: from inorganic and metal crystals to cell membranes and biopolymers. Chem. Rev. 88, 221–242 (1988)
O'Keeffe, M. & Hyde, B. G. Crystal Structures I: Patterns and Symmetry 289–380 (Mineralogical Society of America, Washington DC, 1996)
O'Keeffe, M. Tiling by numbers. Nature 400, 617–618 (1999)
Férey, G. Building units design and scale chemistry. J. Solid State Chem. 152, 37–48 (2000)
Plévert, J., Gentz, T. M., Groy, T. L., O'Keeffe, M. & Yaghi, O. M. Layered structures constructed from new linkages of Ge7(O,OH,F)19 clusters. Chem. Mater. 15, 714–718 (2003)
Hriljac, J. A., Eddy, M. M., Cheetham, A. K., Donohue, J. A. & Ray, G. J. Powder neutron diffraction and 29Si MAS NMR studies of siliceous zeolite-Y. J. Solid State Chem. 106, 66–72 (1993)
Ravikovitch, P. I.,, Haller, G. L. & Neimark, A. V. Density functional theory model for calculating pore size distributions: pore structure of nanoporous catalysts. Adv. Colloid Interf. Sci. 76–77, 203–226 (1998)
Lu, Q., Gao, F., Li, Y., Zhou, Y. & Zhao, D. Synthesis of germanium oxide mesostructures with a new intermediate state. Micropor. Mesopor. Mater. 56, 219–225 (2002)
Connolly, M. L. Solvent accessible surfaces of proteins and nucleic acids. Science 221, 709–713 (1983)
Acknowledgements
We thank S. Lidin for help and discussions, A. Chizmeshya for the volume data reported in Table 1, K. E. Christensen for checking ion-exchanged samples and A. Garcia-Bennett for the N2 isotherm. L. Q. Tang and E. Karlsson participated in the synthesis. The project is supported by the Swedish Science Research Council. M.O'K. acknowledges support from the US National Science Foundation. X.D.Z. is a Research Fellow of the Royal Swedish Academy of Sciences, supported by a grant from the Alice and Knut Wallenberg Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The additional crystallographic data for SU-M (CCDC-278829) and SU-MB (CCDC-278830) can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figures and Supplementary Tables
This file contains Supplementary Figures S1–S4 and Supplementary Tables S1–S4. (DOC 539 kb)
Supplementary Video S1
This movie shows the pore structures and gyroidal channels of SU-M (left) and SU-MB (right) in one unit cell. They are calculated from the structure factors of all reflections with d-values large than 12 Å (four unique ones for SU-M and 7 unique ones for SU-MB) obtained from the framework structures. The red side is towards the pore and the green side is towards the framework wall. In SU-M, two gyroidal channels with opposite chirality are present, whereas in SU-MB, one of them is filled. (GIF 4323 kb)
Rights and permissions
About this article
Cite this article
Zou, X., Conradsson, T., Klingstedt, M. et al. A mesoporous germanium oxide with crystalline pore walls and its chiral derivative. Nature 437, 716–719 (2005). https://doi.org/10.1038/nature04097
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04097
This article is cited by
-
Heteropolyacid supported on ionic liquid decorated hierarchical faujasite zeolite as an efficient catalyst for glycerol acetalization to solketal
Scientific Reports (2023)
-
Hexagulation numbers: the magic numbers of equal spheres on triply periodic minimal surfaces
Structural Chemistry (2017)
-
A zeolite family with expanding structural complexity and embedded isoreticular structures
Nature (2015)
-
Extreme biomimetic approach for developing novel chitin-GeO2 nanocomposites with photoluminescent properties
Nano Research (2015)
-
The ITQ-37 mesoporous chiral zeolite
Nature (2009)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.