Abstract
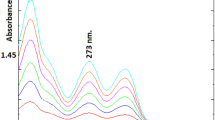
A NUMBER of authors have now reported the occurrence of “pink spot” in the urine of schizophrenics1. Its identification as β-3,4-dimethoxyphenylethylamine (DMPE) rests on paper chromatographic evidence and a melting point provided by Friedhoff and Van Winkle2 together with the gas chromatographic evidence of Sen and McGeer3. The pink spot produced from DMPE by successive treatments with ninhydrin and Ehrlich's reagent is evanescent and some 5–10 µg are required on the paper after chromatography in order to be certain of its occurrence. Drug metabolites can. seriously interfere with its determination by this technique. We find that DMPE is readily detected by its reaction with formaldehyde on paper4, and that it can be separated by high voltage electrophoresis from urinary metabolites of ‘Largactil’ (2,000 mg/day), ‘Stelazine’ (540 mg/day), ‘Nardil’, ‘Disipal’ (300 mg/day), barbiturates and haloperidol (2.25 mg/day). We have examined urines from patients during treatment with these drugs (at the doses quoted), and find that their metabolites give no fluorescence with formaldehyde and do not interfere with the formaldehyde fluorescence of added DMPE. (‘Tofranil’ metabolites, however, had a native fluorescence which may interfere at high doses.) Even when overloading of the paper has caused tailing of the DMPE and overlap with metabolite spots, the fluorescence still develops normally. The fluorophore can be eluted with 0.4 N sodium hydroxide in methanol, and after acidification with hydrochloric acid it can be measured quantitatively in a fluorimeter at 360 mµ activation/460 mµ fluorescence. In case of tailing on the electrophoresis paper, the eluted fluorophore can be re-run on thin layer plates of silica gel using n-butanol : water : acetic acid (60 : 20 : 20), or isopropanol: methyl acetate : water : concentrated ammonia (35: 45: 15: 5), in order to separate it from traces of drug metabolites or other urinary constituents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
For review of the literature see Lancet, ii, 1169 (1965).
Friedhoff, A. J., and van Winkle, E., J. Nerv. Ment. Dis., 135, 550 (1962); Nature, 202, 520 (1964).
Sen, N. P., and McGeer, P. L., Biochem. Biophys. Res. Commun., 14, 227 (1964).
Bell, C. E., and Somerville, A. R., Biochem. J., 98, 1c (1966).
Bourdillon, R. E., Clarke, C. A., Ridges, A. Pauline, Sheppard, P. M., Harper, P., and Leslie, Shirley A., Nature, 208, 453 (1965).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BELL, C., SOMERVILLE, A. Identity of the “Pink Spot”. Nature 211, 1405–1406 (1966). https://doi.org/10.1038/2111405a0
Issue Date:
DOI: https://doi.org/10.1038/2111405a0
This article is cited by
-
Biochemical Research in Schizophrenia
Nature (1971)
-
Urine Volume and Pink Spots in Schizophrenia and Health
Nature (1969)
-
Urinary Metabolites in Parkinson's Disease
Nature (1968)
-
Nor2Chlorpromazine Sulphoxide and 3,4-Dimethoxyphenethylamine
Nature (1968)
-
Identity of a Urinary “Pink Spot” in Schizophrenia and Parkinson's Disease
Nature (1967)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.