Abstract
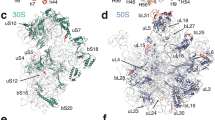
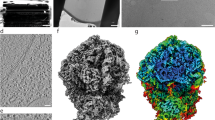
FUNCTIONAL heterogeneity of ribosomal proteins from Escherichia coli is extensive and in vitro polypeptide synthesis requires at least six different protein fractions1. The structural heterogeneity of ribosomal proteins is even more impressive; on gel electrophoresis of 70S ribosomal protein from E. coli about thirty protein bands separate and opinion favours the presence of at least as many distinct proteins2–10. Little is known, however, about the extent to which the primary structure of these proteins is related. In this communication a comparison between the amino-acid composition and tryptic fingerprints of eight simplified protein fractions, isolated from 70S ribosomal proteins of E. coli, will be made. In addition the purification and some properties of three single ribosomal proteins, one acidic and two basics, will be presented. Fig. 1 gives the results of applying 1 g 70S ribosomal protein (prepared using glacial acetic acid2,10) to a column of CM cellulose. Four different peaks were reproducibly resolved. The electrophoresis patterns on polyacrylamide gels (15 per cent) of selected fractions are shown in Fig. 2. As Table 1 shows, the amino-acid compositions of these fractions, determined on a Technicon autoanalyser, are quite distinct. Each fraction contains at least one amino-acid, the percentage of which is substantially greater or less than the percentage of that amino-acid in any other fraction. The relative difference in the contents of basic over acidic amino-acid residues increases regularly from a value of −7 mole per cent to 12 mole per cent. Tryptic fingerprints confirm the presence of extensive differences in primary structure between each of these fractions; with the exception of fractions 1a and 1b, each fraction shows a different tryptic fingerprint with strong spots uniquely missing or present on comparison with any of the other fingerprints (Fig. 3).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Nomura, M., and Traub, P., Proc. Symp. Rutgers (in the press, 1967).
Waller, J. P., and Harris, J. I., Proc. US Nat. Acad. Sci., 47, 18 (1961).
Spitnik-Elson, P., Biochim. Biophys. Acta, 74, 105 (1963).
Waller, J. P., J. Mol. Biol., 10, 319 (1964).
Spitnik-Elson, P., Biochim. Biophys. Acta, 80, 594 (1964).
Leboy, P., Cox, E. C., and Flaks, J. G., Proc. US Nat. Acad. Sci., 52, 1367 (1964).
Möller, W., Abstracts Second International Biophysics Congress, 177, Vienna (1966).
Traut, R. R., J. Mol. Biol., 21, 571 (1966).
Möller, W., and Chrambach, A., J. Mol. Biol., 23, 377 (1967).
Möller, W., and Widdowson, J., J. Mol. Biol., 24 (1967).
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J., J. Biol. Chem., 193, 265 (1951).
Clegg, J. B., Naughton, M. A., and Weatherall, D. J., J. Mol. Biol., 19, 91 (1966).
Gray, W. R., Methods in Enzymology, Enzyme Structure (Academic Press, New York, in the press).
Traut, R. R., and Monro, R. E., J. Mol. Biol., 10, 63 (1964).
Zamir, A., Leder, P., and Elson, D., Proc. US Nat. Acad. Sci., 56, 1794 (1966).
Watson, J. D., Molecular Biology of the Gene (Benjamin, Washington, 1966).
Traut, R. R., Moore, P. B., Delius, H., Noller, H., and Tissières, A., Proc. US Nat. Acad. Sci., 57, 1294 (1967).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
MÖLLER, W., CASTLEMAN, H. Primary Structure Heterogeneity in Ribosomal Proteins from Escherichia coli. Nature 215, 1293–1295 (1967). https://doi.org/10.1038/2151293a0
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1038/2151293a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.