Abstract
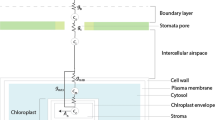
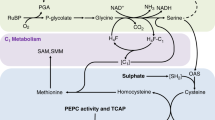
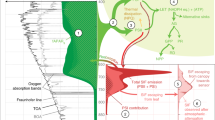
In all green plants both HSO−3 and SO42− are reduced during photosynthesis, and many species can emit the product as H2S gas. Plants will emit H2S after fumigation with acute doses of SO2 gas1,2, irrigation with 5% K2SO4 solutions3, or immersion of roots in HSO−3 and SO42− solutions4. If such emissions of H2S by plants occur in nature, then this process adds sulphur to the atmosphere and contributes to the global sulphur budget. Oxidation of biogenic H2S in the atmosphere may also contribute to the formation of acid rain. We report here that irradiated plants with roots immersed in HSO−3 and SO42− solutions at concentrations found in nature emit H2S from their leaves, and that the fractionation of stable isotopes of sulphur during H2S emission may be useful for identifying atmospheric sulphur that has been generated by photosynthetic reduction of sulphur.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Decormis, L. C. r. hebd Séanc Acad. Sci. D 266, 683–685 (1968).
DeCormis, L. Proc. Ist Eur. Congr. on the Influence of Air Pollution on Plants and Animals, Wageningen, 75–78 (1969).
Spaleny, J. Pl. Soil. 48, 557–563 (1977).
Wilson, L. G., Bressan, R. A. & Filner, P. Pl. Physiol. 61, 184–189 (1978).
Anderson, J. W. Sulphur in Biology Studies in Biology no. 101. (Arnold, London, 1978).
Likens, G. E. & Bormann, F. H. Science 184, 1176–1179 (1974).
Thode, H. G., Monster, J. & Dunford, H. B. Geochim. cosmochim. Acta 25, 159–174 (1961).
Schmidt, A. & Trebst, A. Biochim. biophys. Acta 180, 529–535 (1969).
Trebst, A. & Schmidt, A. Prog. Photosyn. Res. 3, 1510–1516 (1969).
Kellogg, W. W., Cadle, R. D., Allen, E. R., Lazrus, A. L. & Martell, E. A. Science 175, 587–596 (1972).
Nguyen, B. D., Gaudry, A., Bonsang, B. & Lambert, G. Nature 275, 637–639 (1978).
Whittaker, R. H. Communities and Ecosystems, 83 (Macmillan, New York, 1970).
Odum, E. P. Fundamentals of Ecology, 376 (Saunders, Philadelphia, 1971).
Kemp, A. L. W. & Thode, H. G. Geochim. cosmochim. Acta 32, 71–91 (1958).
Harrison, A. G. & Thode, H. G. Trans. Faraday Soc. 53, 1648–1651 (1957).
Krouse, H. R. Nature 265, 45–46 (1977).
Winner, W. E., Bewley, J. D., Brown, H. M. & Krouse, H. R. Oecologia 36, 351–361 (1978).
Case, J. W. & Krouse, H. R. Oecologia 44, 248–257 (1980).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Winner, W., Smith, C., Koch, G. et al. Rates of emission of H2S from plants and patterns of stable sulphur isotope fractionation. Nature 289, 672–673 (1981). https://doi.org/10.1038/289672a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/289672a0
This article is cited by
-
Hydrogen sulfide alleviated chromium toxicity in wheat
Biologia plantarum (2010)
-
Hydrogen sulfide protects soybean seedlings against drought-induced oxidative stress
Acta Physiologiae Plantarum (2010)
-
Hydrogen sulfide counteracts chlorophyll loss in sweetpotato seedling leaves and alleviates oxidative damage against osmotic stress
Plant Growth Regulation (2009)
-
Sulphur isotope ratios in sulphate and oxygen isotopes in water from a small watershed in Central Sweden
Hydrobiologia (1992)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.