Abstract
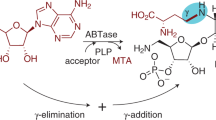
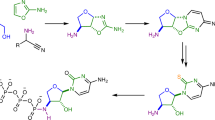
ADENINE thiomethyl pentoside is a sulphur-containing nucleoside first isolated from yeast1. On hydrolysis it yields adenine and a thio-pentose2 which was thought to be a ketose bearing a methylthio or methoxy substituent3 but later recognized as an aldose4. Periodate oxidation studies on the nucleoside, the thio-sugar, its osazone and the thio-pentitol obtained from it by reduction5,6, indicated a 5-methyl-thio group, while the positive Böeseken test for cis-1 : 2 glycols favoured the ribose configuration. The osazone of the thio-sugar was identical with that of 5-methylthio-d-arabinose; but the two sugars themselves were not identical6. The nucleoside must then be a derivative of 5-methylthio-d-ribose, and spectroscopic evidence favoured N9 for the position of attachment of sugar to purine7. The configuration about the glycosidic centre was unknown. On the basis of this evidence, formula (I) is considered probable for adenine thiomethyl pentoside.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Mandel and Dunham, J. Biol. Chem., 11, 85 (1912).
Suzuki, Ohdake and Mori, Biochem. Z., 154, 278 (1924).
Levene and Sobotka, J. Biol. Chem., 65, 551 (1925). Sobotka, ibid., 69, 267 (1926).
Wendt, Z. physiol. Chem., 272, 152 (1942).
Satoh and Makino, Nature, 165, 769 (1950).
Weygand, Trauth and Löwenfeld, Ber., 83, 563 (1950).
Falconer and Gulland, J. Chem. Soc., 1912 (1937).
Levene and Tipson, J. Biol. Chem., 111, 313 (1935).
Kuhn and Henkel, Z. physiol. Chem., 269, 41 (1941).
Suzuki, Ohdake and Mori, Biochem. Z., 162, 413 (1925).
Davoll, Lythgoe and Todd, J. Chem. Soc., 833 (1946); 967 (1948). Lythgoe, Smith and Todd, ibid., 355 (1947) inter al.
Levene and Tipson, J. Biol. Chem., 121, 146 (1937).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BADDILEY, J., TRAUTH, O. & WEYGAND, F. Structure and Synthesis of Adenine Thiomethyl Pentoside; Synthesis of 5′-Methylthio Inosine. Nature 167, 359–360 (1951). https://doi.org/10.1038/167359b0
Issue Date:
DOI: https://doi.org/10.1038/167359b0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.