Abstract
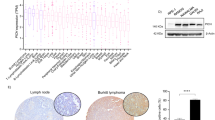
Mucosa-associated lymphoid tissue (MALT) lymphomas most frequently involve the gastrointestinal tract and are the most common subset of extranodal non-Hodgkin lymphoma1 (NHL). Here we describe overexpression of BCL10 , a novel apoptotic signalling gene that encodes an amino-terminal caspase recruitment domain (CARD; ref. 2), in MALT lymphomas due to the recurrent t(1;14)(p22;q32) (ref. 3). BCL10 cDNAs from t(1;14)-positive MALT tumours contained a variety of mutations, most resulting in truncations either in or carboxy terminal to the CARD. Wild-type BCL10 activated NF-κB but induced apoptosis of MCF7 and 293 cells. CARD-truncation mutants were unable to induce cell death or activate NF-κB, whereas mutants with C-terminal truncations retained NF-κB activation but did not induce apoptosis. Mutant BCL10 overexpression might have a twofold lymphomagenic effect: loss of BCL10 pro-apoptosis may confer a survival advantage to MALT B-cells, and constitutive NF-κB activation may provide both anti-apoptotic and proliferative signals mediated via its transcriptional targets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Zucca, E., Roggero, E. & Pileri, S. B-cell lymphoma of MALT type: a review with special emphasis on diagnostic and management problems of low-grade gastric tumours. Br. J. Haematol. 100, 3– 14 (1998).
Hofmann, K., Bucher, P. & Tschopp, J. The CARD domain: a new apoptotic signaling motif. Trends Biochem. Sci. 22, 155–156 (1997).
Wotherspoon, A.C., Pan, L., Diss, T.C. & Isaacson, P.G. Cytogenetic study of B-cell lymphoma of mucosa-associated lymphoid tissue. Cancer Genet. Cytogenet. 58, 35–38 (1992).
Offit, K., Jhanwar, S.C., Ladanyi, M., Filippa, D.A. & Chaganti, R.S. Cytogenetic analysis of 434 consecutively ascertained specimens of non-Hodgkin's lymphoma: correlations between recurrent aberrations, histology, and exposure to cytotoxic treatment. Genes Chromosomes Cancer 3, 189–201 (1991).
Tilly, H. et al. Prognostic value of chromosomal abnormalities in follicular lymphoma. Blood 84, 1043–1049 (1994).
Schlegelberger, B. et al. Cytogenetic findings and results of combined immunophenotyping and karyotyping in Hodgkin's disease. Leukemia 8, 72–80 (1994).
Telford, E.A.R., Watson, M.S., Aird, H.C., Perry, J. & Davison, A.J. The DNA sequence of equine herpesvirus 2. J. Mol. Biol. 249, 520–528 (1995).
Cesarman, E. & Knowles, D.M. Kaposi's sarcoma-associated herpesvirus: a lymphotropic human herpesvirus associated with Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. Semin. Diagn. Pathol. 14, 54–66 (1997).
Chou, J.J., Matsuo, H., Duan, H. & Wagner, G. Solution structure of the RAIDD CARD and model for CARD/CARD interaction in caspase-2 and caspase-9 recruitment. Cell 94, 171– 180 (1998).
Yan, M., Lee, J., Schilbach, S., Goddard, A. & Dixit, V. mE10, a novel CARD-containing proapoptotic molecule. J. Biol. Chem. 274, 10287– 10292 (1999).
Yin, C., Knudson, M., Korsmeyer, S.J. & Van Dyke, T. Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature 385, 637–640 ( 1997).
Storb, U. Progress in understanding the mechanism and consequences of somatic hypermutation. Immunol. Rev. 162, 5–11 (1998).
Shen, H.M., Peters, A., Baron, B., Zhu, X. & Storb, U. Mutation of BCL-6 gene in normal B cells by the process of somatic hypermutation of Ig genes. Science 280, 1750–1752 (1998).
Sonenshein, G.E. Rel/NF-κB transcription factors and the control of apoptosis. Semin. Cancer Biol. 8, 113–119 (1997).
Wang, C.Y., Mayo, M.W., Korneluk, R.G., Goeddel, D.V. & Baldwin, A.S. Jr NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281, 1680–1683 (1998).
Snow, E.C. The role of c-myc during normal B cell proliferation, and as B cells undergo malignant transformation. Curr. Top. Microbiol. Immunol. 224, 211–220 (1997).
Mayo, M.W. et al. Requirement of NF-κB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science 278, 1812–1815 (1997).
Besancon, F., Atfi, A., Gespach, C., Cayre, Y.E. & Bourgeade, M.F. Evidence for a role of NF-κB in the survival of hematopoietic cells mediated by interleukin 3 and the oncogenic TEL/platelet-derived growth factor receptor β fusion protein. Proc. Natl Acad. Sci. USA 95, 8081–8086 ( 1998).
Reuther, J.Y., Reuther, G.W., Cortez, D., Pendergast, A.M. & Baldwin, A.S. Jr A requirement for NF-κB activation in Bcr-Abl-mediated transformation. Genes Dev. 12, 968–981 ( 1998).
Lee, H. et al. Role of the Rel-family of transcription factors in the regulation of c-myc gene transcription and apoptosis of WEHI 231 murine B-cells. Curr. Top. Microbiol. Immunol. 194, 247– 255 (1995).
Baeuerle, P.A. & Baltimore, D. NF-κB: ten years after. Cell 87, 13– 20 (1996).
Gerondakis, S., Grumont, R., Rourke, I. & Grossmann, M. The regulation and roles of Rel/NF-κB transcription factors during lymphocyte activation. Curr. Opin. Immunol. 10, 353– 359 (1998).
Luque, I. & Gelinas, C. Rel/NF-κB and IκB factors in oncogenesis. Semin. Cancer Biol. 8, 103 –111 (1997).
Willis, T.G. et al. Bcl10 is involved in t(1;14)(p22;q32) of MALT B cell lymphoma and mutated in multiple tumor types. Cell 96, 35–45 (1999).
Willis, T.G. et al. Rapid molecular cloning of rearrangements of the IGHJ l ocus using long-distance inverse polymerase chain reaction. Blood 90, 2456–2464 ( 1997).
Siebert, R. et al. Application of interphase fluorescence in situ hybridization for the detection of the Burkitt translocation t(8;14)(q24;q32) in B-cell lymphomas. Blood 91, 984– 990 (1998).
McCarthy, J.V., Ni, J. & Dixit, V.M. RIP2 is a novel NF-κB-activating and cell death-inducing kinase. J. Biol. Chem. 273, 16968–16975 (1998).
Acknowledgements
We thank P. Vandiveer, I.S. Herrington and D. Dodson for manuscript preparation; D. Schuster, C. Becher and C. Reinsch for technical assistance; Y. Zhang, I.M. Subero and T. Springer for help with FISH studies; S. Gesk for assistance with analysis of the sequencing data; and J. Cleveland, J. Downing, G. Grosveld, J. Ihle, M. Kastan, A.T. Look and J. van Deursen for their comments regarding the manuscript. This research was supported in part by Cancer Center Support (CORE) Grant CA-27165 from the National Cancer Institute, the American Lebanese Syrian Associated Charities (ALSAC), St. Jude Children's Research Hospital, the Comité du Rhône de la Ligue Contre le Cancer (Lyon France), Deutsche Krebshilfe Grant 10-0992-Schl3, Wilhelm Sander Stiftung Grant 95.003.2 and the Interdisciplinary Center for Clinical Cancer Research, University of Kiel. B.S. holds a Hermann and Lilly Schilling professorship.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, Q., Siebert, R., Yan, M. et al. Inactivating mutations and overexpression of BCL10, a caspase recruitment domain-containing gene, in MALT lymphoma with t(1;14)(p22;q32) . Nat Genet 22, 63–68 (1999). https://doi.org/10.1038/8767
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/8767
This article is cited by
-
Inherited Human BCL10 Deficiencies
Journal of Clinical Immunology (2024)
-
Novel bioinformatic classification system for genetic signatures identification in diffuse large B-cell lymphoma
BMC Cancer (2020)
-
Human BCL10 Deficiency due to Homozygosity for a Rare Allele
Journal of Clinical Immunology (2020)
-
CARD–BCL-10–MALT1 signalling in protective and pathological immunity
Nature Reviews Immunology (2019)
-
The API2–MALT1 fusion exploits TNFR pathway-associated RIP1 ubiquitination to promote oncogenic NF-κB signaling
Oncogene (2014)