Abstract
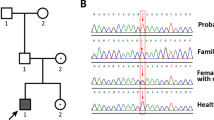
Familial glucocorticoid deficiency (FGD), or hereditary unresponsiveness to adrenocorticotropin (ACTH; OMIM 202200), is an autosomal recessive disorder resulting from resistance to the action of ACTH on the adrenal cortex, which stimulates glucocorticoid production. Affected individuals are deficient in cortisol and, if untreated, are likely to succumb to hypoglycemia or overwhelming infection in infancy or childhood1,2,3. Mutations of the ACTH receptor (melanocortin 2 receptor, MC2R) account for ∼25% of cases of FGD4,5,6,7. FGD without mutations of MC2R is called FGD type 2. Using SNP array genotyping, we mapped a locus involved in FGD type 2 to chromosome 21q22.1. We identified mutations in a gene encoding a 19-kDa single–transmembrane domain protein8, now known as melanocortin 2 receptor accessory protein (MRAP). We show that MRAP interacts with MC2R and may have a role in the trafficking of MC2R from the endoplasmic reticulum to the cell surface.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Shepard, T.H., Landing, B.H. & Mason, D.G. Familial Addison's disease; case reports of two sisters with corticoid deficiency unassociated with hypoaldosteronism. AMA J. Dis. Child. 97, 154–162 (1959).
Migeon, C.J. et al. The syndrome of congenital adrenocortical unresponsiveness to ACTH. Report of six cases. Pediatr. Res. 2, 501–513 (1968).
Clark, A.J.L. & Weber, A. Adrenocorticotropin insensitivity syndromes. Endocr. Rev. 19, 828–843 (1998).
Clark, A.J.L., McLoughlin, L. & Grossman, A. Familial glucocorticoid deficiency caused by a point mutation in the ACTH receptor. Lancet 341, 461–462 (1993).
Tsigos, C., Arai, K., Hung, W. & Chrousos, G.P. Hereditary isolated glucocorticoid deficiency is associated with abnormalities of the adrenocorticotropin receptor gene. J. Clin. Invest. 92, 2458–2461 (1993).
Weber, A. et al. Adrenocorticotropin receptor gene mutations in familial glucocorticoid deficiency: relationships with clinical features in four families. J. Clin. Endocrinol. Metab. 80, 65–71 (1995).
Naville, D. et al. Demonstration by transfection studies that mutations in the adrenocorticotropin receptor gene are one cause of the hereditary syndrome of glucocorticoid deficiency. J. Clin. Endocrinol. Metab. 81, 1442–1448 (1996).
Xu, A. et al. Identification of novel putative membrane proteins selectively expressed during adipose conversion of 3T3-L1 cells. Biochem. Biophys. Res. Commun. 293, 1161–1167 (2002).
Génin, E. et al. Linkage of one gene for Familial Glucocorticoid Deficiency type 2 (FGD2) to chromosome 8q and further evidence of heterogeneity. Hum. Genet. 111, 428–434 (2002).
Matsuzaki, H. et al. Parallel genotyping of over 10,000 SNPs using a one-primer assay on a high-density oligonucleotide array. Genome Res. 14, 414–425 (2004).
Gozani, O., Patton, J.G. & Reed, R. A novel set of spliceosome-associated proteins and the essential splicing factor PSF bind stably to pre-mRNA prior to catalytic step II of the splicing reaction. EMBO J. 13, 3356–3367 (1994).
Metherell, L.A. et al. Pseudoexon activation as a novel mechanism for disease resulting in atypical growth hormone insensitivity. Am. J. Hum. Genet. 69, 641–646 (2001).
Boston, B.A. & Cone, R.D. Characterization of melanocortin receptor subtype expression in murine adipose tissues and in the 3T3-L1 cell line. Endocrinology 137, 2043–2050 (1996).
Noon, L.A., Clark, A.J.L. & King, P.J. A peroxisome proliferator-response element in the murine mc2-r promoter regulates its transcriptional activation during differentiation of 3T3-L1 adipocytes. J. Biol. Chem. 279, 22803–22808 (2004).
Grunfeld, C. et al. Characterization of adrenocorticotropin receptors that appear when 3T3-L1 cells differentiate into adipocytes. Endocrinology 116, 113–117 (1985).
Yang, Y.K. Effects of recombinant agouti-signaling protein on melanocortin action. Mol. Endocrinol. 11, 274–280 (1997).
Elias, L.L.K., Weber, A., Pullinger, G.D., Mirtella, A. & Clark, A.J.L. Functional characterization of naturally occurring mutations of the human adrenocorticotropin receptor: poor correlation of phenotype and genotype. J. Clin. Endocrinol. Metab. 84, 2766–2770 (1999).
Noon, L.A. et al. Failed export of the adrenocorticotropin receptor from the endoplasmic reticulum in non-adrenal cells: evidence in support of a requirement for a specific adrenal accessory factor. J. Endocrinol. 174, 17–25 (2002).
McLatchie, L.M. et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393, 333–339 (1998).
Muff, R., Buhlmann, N., Fischer, J.A. & Born, W. An amylin receptor is revealed following co-transfection of a calcitonin receptor with receptor activity modifying proteins-1 or -3. Endocrinology 140, 2924–2927 (1999).
Saito, H., Kubota, M., Roberts, R.W., Chi, Q. & Matsunami, H. RTP family members induce functional expression of mammalian odorant receptors. Cell 119, 679–691 (2004).
O'Connell, J.R. & Weeks, D.E. PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am. J. Hum. Genet. 63, 259–266 (1998).
Abecasis, G.R., Cherny, S.S., Cookson, W.O. & Cardon, L.R. Merlin-rapid analysis of dense genetic maps using sparse gene flow trees. Nat. Genet. 30, 97–101 (2002).
Strauch, K. et al. Parametric and nonparametric multipoint linkage analysis with imprinting and two-locus-trait models: application to mite sensitization. Am. J. Hum. Genet. 66, 1945–1957 (2000).
Chapple, J.P. & Cheetham, M.E. The chaperone environment at the cytoplasmic face of the endoplasmic reticulum can modulate rhodopsin processing and inclusion formation. J. Biol. Chem. 278, 19087–19094 (2003).
Longshaw, V.M., Chapple, J.P., Balda, M.S., Cheetham, M.E. & Blatch, G.L. Nuclear translocation of the Hsp70/Hsp90 organizing protein mSTI1 is regulated by cell cycle kinases. J. Cell. Sci. 117, 701–710 (2004).
Acknowledgements
We thank M. Korbonits for supplying the human tissue RNA panel, S. Chew for advice and support for RNA splicing studies and G. Cooper for providing the Mrap-FLAG expression vector. This work was supported by a grant from the Biotechnology and Biological Sciences Research Council and the 2002 British Society of Paediatric Endocrinology and Diabetes prize awarded to L.A.M. and A.J.L.C. The work was further supported by the German Federal Ministry of Science and Education through the National Genome Research Network (C.B., F.R. and P.N.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Analysis of tissue distribution of genes within the FGD2 critical region. (PDF 281 kb)
Supplementary Fig. 2
In vitro splicing assay demonstrating that mutation of the IVS3 donor splice site impair splicing. (PDF 199 kb)
Supplementary Fig. 3
Alignment of N-terminal region of human, mouse and rat MRAP orthologues and human C6orf117. (PDF 88 kb)
Supplementary Fig. 4
Intracellular mrap co-localized with ER markers. (PDF 92 kb)
Supplementary Table 1
Genes identified within the FGD2 critical region. (PDF 79 kb)
Rights and permissions
About this article
Cite this article
Metherell, L., Chapple, J., Cooray, S. et al. Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat Genet 37, 166–170 (2005). https://doi.org/10.1038/ng1501
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1501
This article is cited by
-
SCARB1 downregulation in adrenal insufficiency with Allgrove syndrome
Orphanet Journal of Rare Diseases (2023)
-
Structural basis of signaling regulation of the human melanocortin-2 receptor by MRAP1
Cell Research (2023)
-
Buckle up! How the nano-seatbelt MRAP1 fastens ACTH in its orthosteric seat
Cell Research (2023)
-
Acute stress response on Atlantic salmon: a time-course study of the effects on plasma metabolites, mucus cortisol levels, and head kidney transcriptome profile
Fish Physiology and Biochemistry (2023)
-
Functional characterization of melanocortin-3 receptor in rainbow trout (Oncorhynchus mykiss)
Fish Physiology and Biochemistry (2022)