Abstract
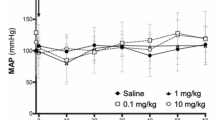
Angiogenesis inhibitors produced by a primary tumor can create a systemic anti-angiogenic environment and maintain metastatic tumor cells in a state of dormancy1,2. We show here that the gallbladder microenvironment modulates the production of transforming growth factor (TGF)-β1, a multifunctional cytokine that functions as an endogenous anti-angiogenic and anti-tumor factor in a cranial window preparation. We found that a wide variety of human gallbladder tumors express TGF-β1 irrespective of histologic type. We implanted a gel impregnated with basic fibroblast growth factor3 or Mz-ChA-2 tumor in the cranial windows of mice without tumors or mice with subcutaneous or gallbladder tumors to study angiogenesis and tumor growth at a secondary site. Angiogenesis, leukocyte–endothelial interaction in vessels and tumor growth in the cranial window were substantially inhibited in mice with gallbladder tumors. The concentration of TGF-β1 in the plasma of mice with gallbladder tumors was 300% higher than that in the plasma of mice without tumors or with subcutaneous tumors. In contrast, there was no difference in the plasma levels of other anti- and pro-angiogenic factors. Treatment with neutralizing antibody against TGF-β1 reversed both angiogenesis suppression and inhibition of leukocyte rolling induced by gallbladder tumors. TGF-β1 also inhibited Mz-ChA-2 tumor cell proliferation. Our results indicate that the production of anti-angiogenesis/proliferation factors is regulated by tumor–host interactions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
O'Reilly, M.S. et al. Angiostatin: A novel angiogenesis inhibitor that mediates the suppression of metastasis by a lewis lung carcinoma. Cell. 79 315–328 (1994).
Volpert, O.V., Lawler, J. & Bouck, N.P. A human fibrosarcoma inhibits systemic angiogenesis and the growth of experimental metastasis via thrombospondin-1. Proc. Natl. Acad. Sci. USA 95, 6343–6348 (1998).
Dellian, M., Witwer, B.P., Salehi, H.A., Yuan, F. & Jain, R.K. Quantitation and physiological characterization of angiogenic vessels in mice: Effect of basic fibroblast growth factor, vascular endothelial growth factor/vascular permeability factor, and host microenvironment. Am. J. Pathol. 149, 59–72 (1996).
Fidler, I.J. Modulation of the organ microenvironment for treatment of cancer metastasis. J. Natl. Cancer Inst. 87, 1588–1592 (1995).
Sckell, A., Safabakhsh, N., Dellian, M. & Jain, R.K. Primary tumor size-dependent inhibition of angiogenesis at a secondary site: An intravital microscopic study in mice. Cancer Res. 58, 5866–5869 (1998).
Robert, B.V. & Sage, E.H. A novel, quantitative model for study of endothelial cell migration and sprout formation within three-dimensional collagen matrices. Microvascular Res. 57, 118–133 (1999).
Müller, G., Behrens, J., Nussbaumer, U., Böhlen, P. & Birchmeier, W. Inhibitory action of transforming growth factor β on endothelial cells. Proc. Natl. Acad. Sci. USA 84, 5600–5604 (1987).
Pepper, M.S., Vassalli, J.D., Orci, L. & Montesnano, R. Biphasic effect of transforming growth factor-β1 on in vitro angiogenesis. Exp. Cell. Res. 204, 356–363 (1993).
Gajdusek, C.M., Luo, Z. & Mayberg, M.R. Basic fibroblast growth factor and Transforming growth factor beta-1: Synergistic mediators of angiogenesis in vitro. J. Cell. Phys. 157, 133–134 (1993).
Pepper, M.S. Transforming growth factor-beta: Vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev. 8, 21–43 (1997).
Yang, E.Y. & Moses, H.L. Transforming growth factor β1-induced changes in cell migration, proliferation, and angiogenesis in the chicken chorioallantoic membrane. J. Cell Biol. 111, 731–741 (1990).
Fajardo, L.F., Prionas, S.D., Kwan, H.H., Kowalski, J. & Allison, A.C. Transforming growth factor β1 induces angiogenesis in vivo with a threshold pattern. Lab. Invest. 74, 600–608 (1996).
Ito, N. et al. Positive correlation of plasma transforming growth factor-beta1 levels with tumor vascularity in hepatocellular carcinoma. Cancer Lett. 89, 45–48 (1995).
Wikstrom, P., Stattin, P., Franck-Lissbrant, I., Damber, J.E. & Bergh, A. Transforming growth factor β1 is associated with angiogenesis, metastasis and poor clinical outcome in prostate cancer. Prostate 37, 19–29 (1998).
Ueki, N. et al. Excessive production of transforming grwoth-factorβ1 can play an important role in the development of tumorigenesis by its action for angiogenesis: validity of neutralizing antibodies to block tumor growth. Biochim. Biophys. Acta 1137, 189–196 (1992).
Engle, S.J. et al. Transforming growth factorβ1 suppresses nonmetastatic colon cancer at an early stage of tumorigenesis. Cancer Res. 59, 3379–3386 (1999).
Norgaard, P., Hougaard, S., Poulsen, H.S. & Spang-Thomsen, M. Transforming growth factor β and cancer. Cancer Treat. Rev. 21, 367–403 (1995).
Theodorescu, D., Caltabiano, M., Greig, R., Rieman, D. & Kerbel, R.S. Reduction of TGF beta activity abrogates growth promoting tumor cell-cell interactions in vivo. J. Cell. Phys. 148, 380–390 (1991).
Carmeliet, P. et al. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394, 485–490 (1998).
Gamble, J.R., Khew-Goodall, Y. & Vadas, M.A. Transforming growth factor-β inhibits E-selectin expression on human endothelial cells. J. Immunol. 150, 4494–4503 (1993).
Walker, R.A. & Dearing, S.J. Transforming growth factor β1 in ductal carcinoma in situ and invasive carcinomas of the breast. Eur. J. Cancer 28, 641–644 (1992).
Nakanishi, Y. et al. The expression of vascular endothelial growth factor and transforming growth factor-β associates with angiogenesis in epithelial ovarian cancer. Int. J. Gynecol. Pathol. 16, 256–262 (1997).
Picon, A., Gold, L.I., Wang, J., Cohen, A. & Friedman, E. A subset of metastatic human colon cancers expresses elevated levels of transforming growth factor β1. Cancer Epidemiol. Biomarkers Prev. 7, 497–504 (1988).
Todoroki, T. et al. Resection combined with intraoperative radiation therapy (IORT) for stage IV (TNM) gallbladder carcinoma. World J. Surg. 15, 357–366 (1991).
Jain, R.K., Schlenger, K., Hockel, M. & Yuan, F. Quantitative angiogenesis assays: progress and problems. Nature Med. 3, 1203–1208 (1997).
Acknowledgements
We thank N. Bouck and C. Koike for the TSP-1 assay; H.C. Lichtenbeld and E. di Tomaso for immunostaining; G. Koenig for ELISA; M. Ancukiewcz for statistical analysis; J. Ang, A. Kadambi, R. Öklü, K. Burton, T. Shioda and B. Seed for comments. This work was supported by National Cancer Institute-Outstanding Investigator Grant R35-CA56591 to R.K.J.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gohongi, T., Fukumura, D., Boucher, Y. et al. Tumor–host interactions in the gallbladder suppress distal angiogenesis and tumor growth: Involvement of transforming growth factor β1. Nat Med 5, 1203–1208 (1999). https://doi.org/10.1038/13524
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/13524
This article is cited by
-
The bone microenvironment promotes tumor growth and tissue perfusion compared with striated muscle in a preclinical model of prostate cancer in vivo
BMC Cancer (2018)
-
A history of exploring cancer in context
Nature Reviews Cancer (2018)
-
Consensus guidelines for the use and interpretation of angiogenesis assays
Angiogenesis (2018)
-
Factors regulating capillary remodeling in a reversible model of inflammatory corneal angiogenesis
Scientific Reports (2016)
-
3D functional and perfusable microvascular networks for organotypic microfluidic models
Journal of Materials Science: Materials in Medicine (2015)