Abstract
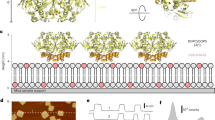
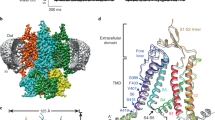
For ligand-gated ion channels, the binding of a ligand to an intracellular or extracellular domain generates changes in transmembrane pore-forming helices, which alters ion flow. The molecular mechanism for this allostery, however, remains unknown. Here we explore the structure and conformational rearrangements of the C-terminal gating ring of the cyclic nucleotide–gated channel CNGA1 during activation by cyclic nucleotides with patch-clamp fluorometry. By monitoring fluorescent resonance energy transfer (FRET) between membrane-resident quenchers and fluorophores attached to the channel, we detected no movement orthogonal to the membrane during channel activation. By monitoring FRET between fluorophores within the C-terminal region, we determined that the C-terminal end of the C-linker and the end of the C-helix move apart when channels open. We conclude that during channel activation, a portion of the gating ring moves parallel to the plasma membrane, hinging toward the central axis of the channel.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hille, B. Ion Channels of Excitable Membranes (Sinauer, Sunderland, Massachusetts, USA, 2001).
Schreiber, M., Yuan, A. & Salkoff, L. Transplantable sites confer calcium sensitivity to BK channels. Nat. Neurosci. 2, 416–421 (1999).
Brauchi, S., Orta, G., Salazar, M., Rosenmann, E. & Latorre, R. A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels. J. Neurosci. 26, 4835–4840 (2006).
Craven, K.B. & Zagotta, W.N. CNG and HCN channels: two peas, one pod. Annu. Rev. Physiol. 68, 375–401 (2006).
Zagotta, W.N. et al. Structural basis for modulation and agonist specificity of HCN pacemaker channels. Nature 425, 200–205 (2003).
Selvin, P.R. Fluorescence resonance energy transfer. Methods Enzymol. 246, 300–334 (1995).
Vogel, S.S., Thaler, C. & Koushik, S.V. Fanciful FRET. Sci. STKE 2006, re2 (2006).
Lakowicz, J.R. Principles of Fluorescence Spectroscopy (Plenum, New York, 1999).
Zheng, J. & Zagotta, W.N. Gating rearrangements in cyclic nucleotide-gated channels revealed by patch-clamp fluorometry. Neuron 28, 369–374 (2000).
Zagotta, W.N. & Siegelbaum, S.A. Structure and function of cyclic nucleotide-gated channels. Annu. Rev. Neurosci. 19, 235–263 (1996).
Craven, K.B. & Zagotta, W.N. Salt bridges and gating in the COOH-terminal region of HCN2 and CNGA1 channels. J. Gen. Physiol. 124, 663–677 (2004).
Johnson, J.P., Jr & Zagotta, W.N. Rotational movement during cyclic nucleotide-gated channel opening. Nature 412, 917–921 (2001).
Hua, L. & Gordon, S.E. Functional interactions between A′ helices in the C-linker of open CNG channels. J. Gen. Physiol. 125, 335–344 (2005).
Matulef, K., Flynn, G.E. & Zagotta, W.N. Molecular rearrangements in the ligand-binding domain of cyclic nucleotide-gated channels. Neuron 24, 443–452 (1999).
Chanda, B., Asamoah, O.K., Blunck, R., Roux, B. & Bezanilla, F. Gating charge displacement in voltage-gated ion channels involves limited transmembrane movement. Nature 436, 852–856 (2005).
Islas, L.D. & Zagotta, W.N. Short-range molecular rearrangements in ion channels detected by tryptophan quenching of bimane fluorescence. J. Gen. Physiol. 128, 337–346 (2006).
Gordon, S.E., Varnum, M.D. & Zagotta, W.N. Direct interaction between amino- and carboxyl-terminal domains of cyclic nucleotide-gated channels. Neuron 19, 431–441 (1997).
Brown, R.L., Snow, S.D. & Haley, T.L. Movement of gating machinery during the activation of rod cyclic nucleotide-gated channels. Biophys. J. 75, 825–833 (1998).
Chanda, B. et al. A hybrid approach to measuring electrical activity in genetically specified neurons. Nat. Neurosci. 8, 1619–1626 (2005).
Fernandez, J.M., Taylor, R.E. & Bezanilla, F. Induced capacitance in the squid giant axon. Lipophilic ion displacement currents. J. Gen. Physiol. 82, 331–346 (1983).
Inouye, S. & Tsuji, F.I. Evidence for redox forms of the Aequorea green fluorescent protein. FEBS Lett. 351, 211–214 (1994).
Ostergaard, H., Henriksen, A., Hansen, F.G. & Winther, J.R. Shedding light on disulfide bond formation: engineering a redox switch in green fluorescent protein. EMBO J. 20, 5853–5862 (2001).
Zheng, J., Varnum, M.D. & Zagotta, W.N. Disruption of an intersubunit interaction underlies Ca2+-calmodulin modulation of cyclic nucleotide-gated channels. J. Neurosci. 23, 8167–8175 (2003).
Wainger, B.J., DeGennaro, M., Santoro, B., Siegelbaum, S.A. & Tibbs, G.R. Molecular mechanism of cAMP modulation of HCN pacemaker channels. Nature 411, 805–810 (2001).
Vemana, S., Pandey, S. & Larsson, H.P. S4 movement in a mammalian HCN channel. J. Gen. Physiol. 123, 21–32 (2004).
Ye, S., Li, Y., Chen, L. & Jiang, Y. Crystal structures of a ligand-free MthK gating ring: insights into the ligand gating mechanism of K+ channels. Cell 126, 1161–1173 (2006).
Jiang, Y. et al. Crystal structure and mechanism of a calcium-gated potassium channel. Nature 417, 515–522 (2002).
Popovych, N., Sun, S., Ebright, R.H. & Kalodimos, C.G. Dynamically driven protein allostery. Nat. Struct. Mol. Biol. 13, 831–838 (2006).
Cordero-Morales, J.F. et al. Molecular determinants of gating at the potassium-channel selectivity filter. Nat. Struct. Mol. Biol. 13, 311–318 (2006).
Kaupp, U.B. et al. Primary structure and functional expression from complementary DNA of the rod photoreceptor cyclic GMP-gated channel. Nature 342, 762–766 (1989).
Gordon, S.E. & Zagotta, W.N. A histidine residue associated with the gate of the cyclic nucleotide-activated channels in rod photoreceptors. Neuron 14, 177–183 (1995).
Hamill, O.P., Marty, A., Neher, E., Sakmann, B. & Sigworth, F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391, 85–100 (1981).
Cha, A. & Bezanilla, F. Structural implications of fluorescence quenching in the Shaker K+ channel. J. Gen. Physiol. 112, 391–408 (1998).
Wolber, P.K. & Hudson, B.S. An analytic solution to the Forster energy transfer problem in two dimensions. Biophys. J. 28, 197–210 (1979).
Flynn, G.E. & Zagotta, W.N. Conformational changes in S6 coupled to the opening of cyclic nucleotide-gated channels. Neuron 30, 689–698 (2001).
Guex, N. & Peitsch, M.C. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18, 2714–2723 (1997).
Long, S.B., Campbell, E.B. & Mackinnon, R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 309, 897–903 (2005).
Acknowledgements
We thank H. Utsugi, S. Cunnington and G. Sheridan for technical assistance, E.R. Liman (University of Southern California) for the pGEMHE oocyte expression vector, R.T. Moon (University of Washington) for the plasmid encoding EGFP-F, W. Almers, K. Craven, G. Flynn, A. Merz, M. Puljung and N. Shuart for comments on the manuscript, and L. Islas for stimulating discussions. This work was supported by the Howard Hughes Medical Institute, a grant from the National Eye Institute of the US National Institutes of Health (EY10329) to W.N.Z. and a postdoctoral fellowship from the Jane Coffin Childs Foundation to J.W.T.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 871 kb)
Rights and permissions
About this article
Cite this article
Taraska, J., Zagotta, W. Structural dynamics in the gating ring of cyclic nucleotide–gated ion channels. Nat Struct Mol Biol 14, 854–860 (2007). https://doi.org/10.1038/nsmb1281
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb1281
This article is cited by
-
A conformational switch in clathrin light chain regulates lattice structure and endocytosis at the plasma membrane of mammalian cells
Nature Communications (2023)
-
Relative positioning of Kv11.1 (hERG) K+ channel cytoplasmic domain-located fluorescent tags toward the plasma membrane
Scientific Reports (2018)
-
New perspectives in cyclic nucleotide-mediated functions in the CNS: the emerging role of cyclic nucleotide-gated (CNG) channels
Pflügers Archiv - European Journal of Physiology (2014)
-
Structure of a KirBac potassium channel with an open bundle crossing indicates a mechanism of channel gating
Nature Structural & Molecular Biology (2012)
-
Mapping the structure and conformational movements of proteins with transition metal ion FRET
Nature Methods (2009)