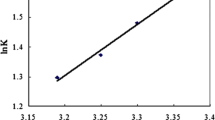
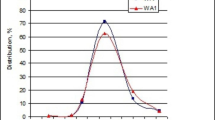
Abstract
The methanol adsorption capacity of an amorphous silica-alumina was measured using an equilibrium technique. The experimental temperature range was of 140 to 260°C and the pure methanol pressure range was 0.1 to 1.2 MPa. A multilayer adsorption was found, also for temperatures above the critical temperature of the adsorbate. Based on the Jovanovic adsorption model, the mean residence times of the adsorbed molecules were calculated. Surprisingly, the heat of adsorption was found to be independent of the temperature in the multilayer adsorption range.
Similar content being viewed by others
Abbreviations
- a :
-
parameter defined by Equation (7)
- a′ :
-
parameter defined by Equation (10) Pa
- a(T):
-
parameter in the Peng-Robinson equation Pa/(mol/m3)2
- b :
-
parameter defined by Equation (8)
- b′ :
-
parameter defined by Equation (11) Pa−1
- b(T):
-
parameter in the Peng-Robinson equation m3 mol−1
- c :
-
constant in the BET equation
- k :
-
Boltzmann constant, being 1.3806 · 10−23 JK−1
- K :
-
characteristic constant in the Peng-Robinson equation
- m :
-
mass of one adsorbate molecule kg
- p :
-
pressure Pa
- q :
-
adsorption capacity g/g
- Q :
-
heat of adsorption J mol−1
- R :
-
gas constant, being 8.314 J mol−1 K−1
- T :
-
absolute temperature K
- V :
-
molar volume m3 mol−1
- x :
-
relative pressure (=p/p 0)
- σ :
-
active molecule area m2
- τ :
-
residence time s
- ω :
-
acentric factor
References
Peng DYu, Robinson DB (1976) Ind Eng Chem Fundam 15(1):59
Hirschfelder JO, Curtiss CF, Bird RB (1966) Molecular Theory of Gases and Liquids, 3rd ed, J Wiley & Sons, New York
Bhattacharyya D, Thodos G (1964) J Chem Eng Data 9:530
Ambrose D, Sprake CHS, Townsend R (1975) J Chem Thermod 7:185
Susic MV, Vucelic DP, Pausak SV, Karaulic DB, MilakovicVucelic V (1969) J Phys Chem 73:1975
Hüttig GF (1948) Monatsh Chemie 78:177
Brunauer S, Emmett P, Teller E (1938) J Am Chem Soc 60:309
Anderson RB (1946) J Am Chem Soc 68:686
Smith RN, Pierce C (1948) J Phys Chem 52:1115
Steele WA (1956) J Phys Chem 25:819
Pickett G (1945) J Am Chem Soc 67:1958
Lunde PJ, Kester FL (1975) Chem Eng Sci 30:1497
Jovanovic DS (1969) Kolloid ZZ Polym 235:1203
Jovanovic DS (1969) Kolloid ZZ Polym 235:1214
Yaronets YA, Yaronets M (1980) Bull Acad Sci USSR Div of Chem Sci 29:870
Jaroniec M, Sokolowski S, Waksmudzki A (1976) Roczn Chemii 50:779
Sokolowski S, Jaroniec M, Waksmudzki A (1976) Roczn Chemii 50:1149
de Boer JH (1968) The Dynamical Character of Adsorption, Oxford University Press, London
Radosz M, Lydersen A (1980) Chem Ing Tech 52:756
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kuczynski, M., van Ooteghem, A. & Westerterp, K.R. Methanol adsorption by amorphous silica alumina in the critical temperature range. Colloid & Polymer Sci 264, 362–367 (1986). https://doi.org/10.1007/BF01418197
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01418197