Summary
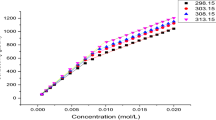
The solubilities of the following compounds in an aqueous sodium dodecyl sulfate solution were determined at 25°, 30°, 35° and 40°C: biphenyl, azobenzene, p-chloroazobenzene, p-aminoazobenzene, p-N,N-dimethylaminoazobenzene and p-nitroazobenzene. From the results the thermodynamic parameters for the transfer of the model compounds from water to SDS micellar environment were calculated. The resulting thermodynamic parameters were not so certain, but suggested that with biphenyl, azobenzene and p-chloroazobenzene which seem to be solubilized in the SDS micellar interior the solubilizing process is a result of a favourable increase in entropy, and that with p-aminoazobenzene, p-N, N-dimethylaminoazobenzene and p-nitroazobenzene which seem to be solubilized in the SDS micellar surface the solubilizing process is a result of a favourable decrease in enthalpy. The favourable increase in entropy was explained in terms of iceberg and the favourable decrease in enthalpy in terms of interfacial energy.
Similar content being viewed by others
References
For instance:Goddard, E. D. andG. C. Benson, Trans. Faraday Soc.52, 409 (1956),Goddard, E. D., C. A. J. Hoeve andG. C. Benson, J. Phys. Chem.61, 593 (1957),Benjamin, L., J. Phys. Chem.68, 3575 (1964).
Wishnia, A., J. Phys. Chem.67, 2079 (1963).
Katayama, A., N. Kuroki andK. Konishi, Sen-i-Gakkaishi20, 256 (1963).
Katayama, A., T. Matsuura, K. Konishi andN. Kuroki, Kolloid-Z. u. Z. Polymere202, 157 (1965).
Dreger, E. E., G. I. Keim, G. D. Miles, L. Shedlovsky andJ. Ross, Ind. Eng. Chem.36, 610 (1944).
Williams, R. J., J. L. Phillips andK. J. Mysels, Trans. Faraday Soc.51, 728 (1955).
Hartley, G. S., J. Chem. Soc, p. 1968 (1938).
Flockhart, B. D., J. Colloid Sci.16, 484 (1961).
Phillips, J. N. andK. J. Mysels, J. Phys. Chem.59, 325 (1955).
McBain, M. E. L., Solubilization (New York 1955).
Elevens, H. B., Chem. Rev.47, 1 (1950).
Takagishi, T.,A. Katayama andN. Kuroki (unpublished).
Frank, H. S. andW. Y. Wen, Discussions Faraday Soc.24, 133 (1957).
Nightingale, E. R., J. Phys. Chem.63, 1381 (1959).
Benjamin. L., J. Phys. Chem.68, 3575 (1964).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Takagishi, T., Katayama, A., Konishi, K. et al. The solubility of disperse dyes in an aqueous sodium dodecyl sulfate solution. Kolloid-Z.u.Z.Polymere 232, 689–693 (1969). https://doi.org/10.1007/BF01500165
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01500165