Summary
The crystal structure of hydrothermally grown NaFe[SeO4]2 was determined by single crystal X-ray diffraction methods: space group C2/m, a = 8.231(1)Å b = 5.425(1)Å, c = 7.176(1)Å, β = 92.44(1)°, V = 320.14Å3, Z = 2; 767 unique data, measured up to 20 = 70° (Mo Kx radiation); R,RW= 0.042, 0.055.
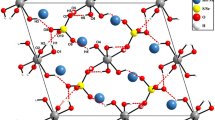
NaFe[SeO4]2 is isotypic with yavapaiite, KFe[SO4]2. FeO6 and NaO6 form chains of edge-sharing distorted octahedra with mean Me-O bond lengths of 2.001Å and 2.496Å, respectively, linked via tetrahedral SeO4 groups [<Se-O) = 1.639Å].
Zusammenfassung
Die Kristallstruktur von hydrothermal gezüchtetem NaFe[SeO4]2 wurde mittels Einkristallröntgenmethoden bestimmt: Raumgruppe C2/m, a = 8.231(1)Å, b = 5.425(1)Å, c = 7.176(1)Å, β = 92.44(1)°, V = 320.14Å3, Z = 2; 767 unabhängige Intensitätsdaten, Meßbereich bis 20 = 70° (Mo Kα-Strahlung); R,RW = 0.042, 0.055.
NaFe[SeO4]2 ist isotyp mit Yavapaiit, KFe[SO4]2. FeO6 und NaO6 bilden Ketten von kantenverknüpften, verzerrten Oktaedern mit mittleren Me-O Bindungslängen von 2.001Å bzw. 2.496Å, die über tetraedrische SeO4 Gruppen verbunden sind [<Se-O> = 1.639Å].
Similar content being viewed by others
References
Anthony JW, McLean WJ, Laughon RB (1972) The crystal structure of yavapaiite: a discussion. Am Mineral 57: 1546–1549
Giester G (1992) The crystal structure of Fe(SeO2OH)(SeO4) · H2O. Mh Chem 123: 957–963
Giester G (1993a) Crystal structure of Fe(III)2(SeO3)3 - H2O. j Sol St Chem: 103
Giester G (1993b) Crystal structure of KFe(SeO3)2. Z Krist (in press)
Giester G (1993c) LiFe3+(Se2O5)2 and its structural relationships to Me2+(Se2O5) compounds. Mh Chem (submitted)
Giester G, Wildner M (1991) Synthesis and crystal structure of monoclinic Fe2(SeO4)3. Mh Chem 122: 617–623
Giester G, Wildner M (1992) The crystal structures of kieserite-type compounds. II. Crystal structures of Me(II)SeO4 - H2O [Me = Mg, Mn, Co, Ni, Zn]. N Jb Min Mh 1992: 135–144
Graeber J, Rosenzweig A (1971) The crystal structure of yavapaiite, KFe(SO4)2, and goldichite, KFe(Sb4)2 - 4H2O. Am Mineral 56: 1917–1933
International Tables for X-ray Crystallography (1974) vol IV. Revised and supplementary Tables.Ibers JA, Hamilton WC (eds) Birmingham, The Kynoch Press
Samaras D, Coing-Boyat J (1970) Affinement de la structure de FeSO4-α. Bull Soc fr Mineral Cristallogr 93: 190–194
Valkonen J, Koskenlinna M (1978) The crystal structure of iron(III) hydrogen biselenite, FeH(SeO3)2. Acta Chem Scand A32: 603–606
Wildner M, Giester G (1988) Crystal structure refinements of synthetic chalcocyanite (CUSO4) and zincosite (ZnSO4). Min Pet 39: 201–209
Wildner M, Giester G (1991) The crystal structures of kieserite-type compounds. 1. Crystal structures of Me(II)SO4 - H2O [Me = Mn, Fe, Co, Ni, Zn]. N Jb Min Mh 1991: 296–306
Zachariasen WH (1967) A general theory of X-ray diffraction in crystals. Acta Cryst 23: 558–564
Author information
Authors and Affiliations
Additional information
Dedicated to Prof. Josef Zemann on the occasion of his 70th birthday
With 3 Figures
Rights and permissions
About this article
Cite this article
Giester, G. Crystal structure of the yavapaiite type compound NaFe[SeO4]2 . Mineralogy and Petrology 48, 227–233 (1993). https://doi.org/10.1007/BF01163100
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01163100