Summary
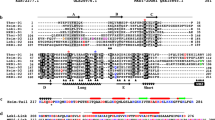
In the primitive red algaCyanidium caldarium RK-1, cytokinesis is controlled by a simple contractile ring, as in animal cells. To clarify the mechanism of formation of the contractile ring, we isolated actin genes and performed an immunocytological study.C. caldarium RK-1 has two actin genes encoding proteins with the same sequence of 377 amino acids. The primary structure indicated that the actin molecules ofC. caldarium RK-1 are typical, despite the fact that the organism is considered to be phylogenetically primitive. We prepared antiserum against aC. caldarium RK-1 actin fusion protein and indirect immunofluorescence staining was performed. In interphase cells, many actin dots were observed in the cytoplasm but none at the future cleavage plane. Prior to cytokinesis, some of these dots appeared and became aligned along the equatorial plane. At the same time, a thin “immature” contractile ring was observed to appear to be formed by connection of the aligned actin dots. This immature contractile ring thickened to nearly its maximum size by the time cytokinesis began. The formation of the contractile ring seemed to be a result of de novo assembly of actin monomers, rather than a result of the accumulation and bundling of pre-existing actin filaments. During the constriction of the contractile ring, no actin dots were observed in the cytoplasm. These observations suggest that actin dots are responsible for the formation of the contractile ring, but are not necessary for its disintegration. Furthermore, immunogold localization specific for actin revealed at electron microscopy level that fine filaments running just beneath the cleavage furrow are, in fact, actin filaments.
Similar content being viewed by others
Abbreviations
- ORF:
-
open reading frame
- IPTG:
-
isopropyl-β-D(−)-thiogalactopyranoside
- SDS-PAGE:
-
sodium dodecyl sulphate-poly-acrylamide gel electrophoresis
- DAPI:
-
4′,6-diamidino-2-phenylindole
References
Adams AEM, Pringle JR (1984) Relationship of actin and tubulin distribution to bud growth in wild-type and morphogeneticmutantSaccharomyces cerevisiae. J Cell Biol 98: 934–945
Allen MB (1959) Studies withCyanidium caldarium, an anomalously pigmented chlorophyte. Arch Microbiol 32: 270–277
Baskin TI, Cande WZ (1991) The structure and function of the mitotic spindle in flowering plants. Annu Rev Plant Physiol Plant Mol Biol 41: 277–315
Blake CCF (1978) Exons encode protein functional units. Nature 277: 598
— (1983) Exons: present from the beginning? Nature 306: 535–537
Cande WZ (1980) A permeabilized cell model for studying cytokinesis using mammalian tissue culture cells. J Cell Biol 87: 326–335
Cao LG, Wang YL (1990a) Mechanism of the formation of contractile ring in dividing cultured animal cells I: recruitment of preexisting actin filaments into the cleavage furrow. J Cell Biol 110: 1089–1096
— — (1990b) Mechanism of the formation of contractile ring in dividing cultured animal cells II: cortical movement of microinjected actin filaments. J Cell Biol 111: 1905–1912
Cavalier-Smith T (1985) Selfish DNA and the origin of introns. Nature 315: 283–284
—, (1991) Intron phylogeny: a new hypothesis. Trends Genet 7: 145–148
Clayton L, Lloyd CW (1985) Actin organization during the cell cycle in meristematic plant cells. Exp Cell Res 156: 231–238
Cresnar B, Mages W, Müller K, Salbaum JM, Schmitt R (1990) Structure and expression of a single actin gene inVolvox carteri. Curr Genet 18: 337–346
Darnell JE (1978) Implications of RNA · RNA splicing in evolution of eukaryotic cells. Science 202: 1257–1260
De Lozanne A, Spudich JA (1987) Disruption of theDictyostelium myosin heavy chain gene by homologous recombination. Science 236: 1086–1091
Doolittle WF (1978) Genes in pieces: were they ever together? Nature 272: 581–582
Drubin DG, Jones HD, Wertman KF (1993) Actin structure and function: roles in mitochondrial organization and morphogenesis in budding yeast and identification of the phalloidin-binding site. Mol Biol Cell 4: 1277–1294
Erba HP, Eddy R, Shows T, Kedes L, Gunning P (1988) Structure, chromosome location, and expression of the human gammaactin gene: differential evolution, location, and expression of the cytoskeletal beta- and gamma-actin genes. Mol Cell Biol 8: 1775–1789
Fidel S, Doonan JH, Morris NR (1988)Aspergillus nidulans contains a single actin gene which has unique intron locations and encodes a γ-actin. Gene 70: 283–293
Fishkind DJ, Wang YL (1995) New horizons for cytokinesis. Curr Opin Cell Biol 7: 23–31
Fornwald JA, Kuncio G, Peng I, Ordahl CP (1982) The complete nucleotide sequence of the chick α-actin gene and its evolutionary relationship to the actin gene family. Nucleic Acids Res 10: 3861–3876
Fujie M, Kuroiwa H, Kawano S, Mutoh S, Kuroiwa T (1994) Behavior of organelles and their nucleoids in the shoot apical meristem during leaf development inArabidopsis thaliana L. Planta 194: 395–405
Fyrberg EA, Mahaffey JW, Bond BJ, Davidson N (1983) Transcripts of the sixDrosophila actin genes accumulate in a stage- and tissue-specific manner. Cell 33: 115–123
Gallwitz D, Sures I (1980) Structure of a split yeast gene: complete nucleotide sequence of the actin gene inSaccharomyces cerevisiae. Proc Natl Acad Sci USA 77: 2546–2550
Hamada H, Petrino MG, Kakunaga T (1982) Molecular structure and evolutionary origin of human cardiac muscle actin gene. Proc Natl Acad Sci USA 79: 5901–5905
Harper DS, Jahn CL (1989) Differential use of termination codons in ciliated protozoa. Proc Natl Acad Sci USA 86: 3252–3256
Hirono M, Endoh H, Okada N, Numata O, Watanabe Y (1987)Tetrahymena actin: cloning and sequencing of theTetrahymena actin gene and identification of its gene product. J Mol Biol 194: 181–192
—, Kumagai Y, Numata O, Watanabe Y (1989) Purification ofTetrahymena actin reveals some unusual properties. Proc Natl Acad Sci USA 86: 75–79
Katoh T, Morita F (1995) Mapping myosin-binding sites on actin probed by peptides that inhibit actomyosin interaction. J Biochem 120: 580–586
Kilmartin JV, Adams AEM (1984) Structural rearrangements of tubulin and actin during the cell cycle of the yeastSaccharomyces. J Cell Biol 98: 922–933
Knecht DA, Loomis WF (1987) Antisense RNA inactivation of myosin heavy chain gene expression inDictyostelium discoideum. Science 236: 1086–1091
Kusakabe T, Hikosaka A, Satoh N (1995) Coexpression and promoter function in two muscle actin gene complexes of different structural organization in the ascidianHalocynthia roretzi. Dev Biol 169: 461–472
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227: 680–685
Mabuchi I (1986) Biochemical aspects of cytokinesis. Int Rev Cytol 101: 175–213
— (1994) Cleavage furrow: timing of emergence of contractile ring actin filaments and establishment of the contractile ring by filament bundling in sea urchin eggs. J Cell Sci 107: 1853–1862
—, Okuno M (1977) The effect of myosin antibody on the division of the starfish blastomeres. J Cell Biol 74: 251–263
—, Tsukita Sh, Tsukita Sa, Sawai T (1988) Cleavage furrow isolated from newt eggs: contraction, organization of the actin filaments, and protein components of the furrow. Proc Natl Acad Sci USA 85: 5966–5970
Melloul D, Aloni B, Calvo J, Yaffe D, Nudel U (1984) Developmentally regulated expression of chimeric genes containing muscle actin DNA sequences in transfected myogenic cells. EMBO J 3: 983–990
Mertins P, Gallwitz D (1987) A single intronless actin gene in the fission yeastSchizosaccharomyces pombe: nucleotide sequence and transcripts formed in homologous and heterologous yeast. Nucleic Acids Res 15: 7369–7379
Miki M, Barden JA, dos Remedios CG, Phillips L, Hambly BD (1987) Interaction of phalloidin with chemically modified actin. Eur J Biochem 165: 125–130
Mita T, Kuroiwa T (1988) Division of plastids by a plastid-dividing ring inCyanidium caldarium. Protoplasma Suppl 1: 133–152
—, Kanbe T, Tanaka T, Kuroiwa T (1986) A ring structure around the dividing plane of theCyanidium caldarium chloroplast. Protoplasma 130: 211–213
Mounier N, Prudhomme JC (1991) Differential expression of muscle and cytoplasmic actin genes during development ofBombyx mori. Insect Biochem 21: 523–533
Nagashima H, Fukuda I (1981) Morphological properties ofCyanidium caldarium and related algae in Japan. Jpn J Phycol 29: 237–242
Nairn CJ, Winesett L, Ferl RJ (1988) Nucleotide sequence of an actin gene fromArabidopsis thaliana. Gene 65: 247–257
Ng R, Abelson JN (1980) Isolation and sequence of the gene for actin inSaccharomyces cerevisiae. Proc Natl Acad Sci USA 77: 3912–3916
Ohta N, Nagashima H, Kawano S, Kuroiwa T (1992) Isolation of the chloroplast DNA and the sequence of thetrnK gene fromCyanidium caldarium strain RK-1. Plant Cell Physiol 33: 657–661
—, Sato N, Kawano S, Kuroiwa T (1994) ThetrpA gene on the plastid genome ofCyanidium caldarium strain RK-1. Curr Genet 25: 357–361
Rappaport R (1986) Establishment of the mechanism of cytokinesis in animal cells. Int Rev Cytol 105: 245–281
Remedies CGD, Moens PDJ (1995) Actin and the actomyosin interface: a review. Biochim Biophys Acta 1228: 99–124
Romans P, Firtel RA (1985) Organization of the actin multigene family ofDictyostelium discoideum and analysis of variability in the protein coding regions. J Mol Biol 186: 321–335
Sanchez F, Tobin SL, Rdest U, Zulauf E, McCarthy BJ (1983) TwoDrosophila actin genes in detail: gene structure, protein structure and transcription during development. J Mol Biol 163: 533–551
Sanger JM, Sanger JW (1980) Banding and polarity of actin filaments in interphase and cleaving cells. J Cell Biol 86: 568–575
Satterwhite LL, Pollard TD (1992) Cytokinesis. Curr Opin Cell Biol 4: 43–52
Schmit AC, Lambert AM (1990) Microinjected fluorescent phalloidin in vivo reveals the F-actin dynamics and assembly in higher plant mitotic cells. Plant Cell 2: 129–138
Schroeder TE (1968) Cytokinesis: filaments in the cleavage furrow. Exp Cell Res 53: 272–276
— (1970) The contractile ring I: fine structure of dividing mammalian (HeLa) cells and the effects of cytochalasin B. Z Zellforsch Mikrosk Anat 109: 431–449
— (1972) The contractile ring II: determining its brief existence, volumetric changes, and vital role in cleavingArbacia eggs. J Cell Biol 53: 419–434
— (1975) Dynamics of the contractile ring. In: Inoué S, Stephens RE (eds) Molecules and cell movement. Raven, New York, pp 305–334
Seckbach J (1996) Biological aspects of the origin of life: open questions in eukaryogenesis. In: Flores JC, Raulin F (eds) Chemical evolution: physics of the origin and evolution of life. Kluwer, Dordrecht, pp 197–213
—, González E, Wainwright IM, Gross W (1992) Peroxisomal function in the Cyanidiophyceae (Rhodophyta): a discussion of phylogenetic relationships and the evolution of microbodies (peroxisomes). Nova Hedwigia 55: 99–109
Selman GG, Perry MM (1970) Ultrastructural changes in the surface layers of the newt's egg in relation to the mechanism of its cleavage. J Cell Sci 6: 207–227
Sugase Y, Hirono M, Kindle KL, Kamiya, R (1996) Cloning and characterization of the actin-encoding gene ofChlamydomonas reinhardtii. Gene 168: 117–121
Suzuki K, Ohta N, Kuroiwa T (1992) Isolation of the cell-nuclear, mitochondrial, and chloroplast DNA from the ultra-small eukaryoteCyanidioschyzon merolae. Protoplasma 171: 80–84
—, Kawazu T, Mit T, Takahashi H, Itoh R, Toda K, Kuroiwa T (1995) Cytokinesis by a contractile ring in the primitive red algaCyanidium caldarium RK-1. Eur J Cell Biol 67: 170–178
Szollosi D (1970) Cortical cytoplasmic filaments of cleaving eggs: a structural element corresponding to the contractile ring. J Cell Biol 44: 192–209
Takahashi H, Takano H, Yokoyama A, Hara Y, Kawano S, Toh-e A, Kuroiwa T (1995) Isolation, characterization and chromosomal mapping of an actin gene from the primitive red algaCyanidioschyzon merolae. Curr Genet 28: 484–490
—, Kuroiwa H, Miyagishima S, Toda K, Itoh R, Kuroiwa T (1997) Improved procedure for immunogold electron microscopy: rapid-freeze substitution with absolute acetone. Cytologia 62: 303–308
Tilney LG, Marsland D (1969) A fine structural analysis of cleavage induction and furrowing in the eggs ofArbacia punctulata. J Cell Biol 42: 170–184
Usui N, Yoneda M (1982) Ultrastructural basis of the tension increase in sea urchin eggs prior to cytokinesis. Dev Growth Differ 24: 453–465
Vandekerckhove J, Deboben A, Nassal M, Wieland T (1985) The phalloidin binding site of F-actin. EMBO J 4: 2815–2818
Weber K, Kabsch W (1994) Intron positions in actin genes seem unrelated to the secondary structure of the protein. EMBO J 13: 1280–1286
Wick SM (1991) Spatial aspect of cytokinesis in plant cells. Curr Opin Cell Biol 3: 253–260
Wieland T (1977) Modification of actins by phallotoxins. Naturwissenschaften 64: 303–309
Wulf E, Deboden A, Bautz FA, Faulstich H, Wieland TH (1979) Fluorescent phallotoxin, a tool for the visualization of cellular actin. Proc Natl Acad Sci USA 76: 4498–4502
Yumura S (1996) Spatial distribution of fluorescently labeled actin in livingDictyostelium amoebae. Cell Struct Funct 21: 189–197
Zang D, Wadsworth P, Hepler PK (1993) Dynamics of microfilaments are similar, but distinct from microtubules during cytokinesis in living, dividing plant cells. Cell Motil Cytoskeleton 24: 151–155
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Takahashi, H., Takano, H., Kuroiwa, H. et al. A possible role for actin dots in the formation of the contractile ring in the ultra-micro algaCyanidium caldarium RK-1. Protoplasma 202, 91–104 (1998). https://doi.org/10.1007/BF01280878
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01280878