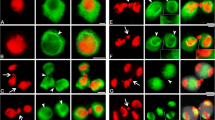
Summary
Cytokinesis following asymmetrical pollen mitosis was studied in the slipper orchidCypripedium fasciculatum using techniques of immunofluorescence, confocal laser scanning, and transmission electron microscopy. Data from stereo reconstructions of double labelled preparations (microtubules/nuclei) show that the contribution of residual spindle fibers to development of the interzonal array is minor; rather, new populations of microtubules are nucleated in association with the two groups of anaphase chromosomes. As kinetochores reach the poles, trailing arms of the chromosomes and nonkinetochore microtubules are displaced outward in the equatorial zone and by early telophase the interzone is left virtually free of microtubules. The interzonal apparatus has its origin in a massive proliferation of microtubules from the polar regions and surfaces of contracting chromosomes. Each polar region appears as a hub from which microtubules radiate in a spoke-like configuration and numerous tufts of microtubules appear to emanate from margins of the chromosomes themselves. These newly organized arrays of microtubules extend to the equatorial region where they interact to form the interzonal apparatus. Increasing organization of microtubules in the interzone results in development of a typical phragmoplast configuration consisting of opposing cone-like bundles of microtubules bisected by an unstained equatorial line.
Similar content being viewed by others
References
Asada T, Sonobe S, Shibaoka H (1991) Microtubule translocation in the cytokinetic apparatus of cultured tobacco cells. Nature 350: 238–241
Bajer AS (1968) Fine structure studies on phragmoplast and cell plate formation. Chromosoma 24: 383–417
—, Molè-Bajer J (1982) Asters, poles, and transport properties within spindlelike microtubule arrays. Cold Spring Harb Symp Quant Biol 46: 263–283
— — (1986) Reorganization of microtubules in endosperm cells and cell fragments of the higher plantHaemanthus in vivo. J Cell Biol 102: 263–281
Brown RC, Lemmon BE (1989) Minispindles and cytoplasmic domains in microsporogenesis in orchids. Protoplasma 148: 26–32
— — (1991a) Pollen development in orchids. 3. A novel generative pole microtubule system predicts unequal pollen mitosis. J Cell Sci 99: 273–281
— — (1991b) Pollen development in orchids. 5. A generative cell domain involved in spatial control of the hemispherical cell plate. J Cell Sci 100: 559–565
— — (1991c) The cytokinetic apparatus in meiosis: control of division plane in the absence of a preprophase band of microtubules. In: Lloyd CW (ed) The cytoskeletal basis of plant growth and form. Academic Press, London, pp 259–273
— — (1992a) Cytoplasmic domain: a model for spatial control of cytokinesis in reproductive cells of plants. Electron Microsc Soc Am Bull 22: 48–53
— — (1992b) Control of division plane in normal and griseofulvin-treated microsporocytes ofMagnolia. J Cell Sci 103: 1031–1038
— — (1994) Pollen mitosis in the slipper orchidCypripedium fasciculatum. Sex Plant Reprod 7: 87–94
— — (1996) Nuclear cytoplasmic domains, microtubules and organelles in microsporocytes of the slipper orchidCypripedium californicum A. Gray dividing by simultaneous cytokinesis. Sex Plant Reprod 9: 145–152
Burns-Balogh P, Hesse M (1988) Pollen morphology of the cypripedioid orchids. Plant Syst Evol 158: 165–182
Busby CH, Gunning BES (1989) Development of the quadripolar meiotic apparatus inFunaria spore mother cells: analysis by means of anti-microtubule drug treatments. J Cell Sci 93: 267–277
De Mey J, Lambert A-M, Bajer AS, Moremans M, de Brabander M (1982) Visualization of microtubules in interphase and mitotic plant cells ofHaemanthus endosperm with the immuno-gold staining method. Proc Natl Acad Sci USA 79: 1898–1902
Euteneuer U, Jackson W, McIntosh JR (1982) Polarity of spindle microtubules inHaemanthus endosperm. J Cell Biol 94: 644–653
Falconer MM, Seagull RW (1987) Amiprophos-methyl (APM): a rapid, reversible, anti-microtubule agent for plant cell cultures. Protoplasma 136: 118–124
Galatis B, Apostolakos P (1991) Patterns of microtubule reappearance in root cells ofVigna sinensis recovering from a colchicine treatment. Protoplasma 160: 131–143
Gunning BES (1982) The cytokinetic apparatus: its development and spatial regulation. In: Lloyd CW (ed) The cytoskeleton in plant growth and development. Academic Press, London, pp 229–292
— (1992) Use of confocal microscopy to examine transitions between successive microtubule arrays in the plant cell division cycle. In: Shibaoka H (ed) Proceedings of the VII International Symposium on the cellular basis of growth and development in plants. Osaka University Press, Osaka, pp 145–155
Huang B-Q, Sheridan WF (1994) Female gametophyte development in maize: microtubular organization and embryo sac polarity. Plant Cell 6: 845–861
Inoué S, Bajer AS (1961) Birefringence in endosperm mitosis. Chromosoma 12: 48–63
Jungers V (1931) Figures caryocinétiques et cloisonnement du protoplasme dans l'endosperme d'Iris. Cellule 40: 292–353
Kakimoto T, Shibaoka H (1988) Cytoskeletal ultrastructure of phragmoplast-nuclei complexes isolated from cultured tobacco cells. Protoplasma Suppl 2: 95–103
Lambert A-M, Bajer AS (1972) Dynamics of spindle fibers and microtubules during anaphase and phragmoplast formation. Chromosoma 39: 101–144
Molè-Bajer J, Bajer AS, Inoué S (1988) Three-dimensional localization and redistribution of F-actin in higher plant mitosis and cell plate formation. Cell Motil Cytoskeleton 10: 217–228
O'Brien TP (1972) The cytology of cell-wall formation in some eukaryotic cells. Bot Rev 38: 87–118
Olsen O-A, Brown RC, Lemmon BE (1995) Pattern and process of wall formation in developing endosperm. BioEssays 17: 803–812
Palevitz BA (1988) Microtubular fir-trees in mitotic spindles of onion roots. Protoplasma 142: 74–78
Panteris E, Galatis B, Apostolakos P (1991) Patterns of cortical and perinuclear microtubule organization in meristematic root cells ofAdiantum capillus veneris. Protoplasma 165: 173–188
Schmit A-C, Vantard M, de Mey J, Lambert A-M (1983) Aster-like microtubule centers establish spindle polarity during interphase-mitosis transition in higher plant cells. Plant Cell Rep 2: 285–288
Schopfer CR, Hepler PK (1991) Distribution of membranes and the cytoskeleton during cell plate formation in pollen mother cells ofTradescantia. J Cell Sci 100: 717–728
Smirnova EA, Bajer AS (1994) Microtubule converging centers and reorganization of the interphase cytoskeleton and the mitotic spindle in higher plantHaemanthus. Cell Motil Cytoskeleton 27: 219–233
Staehelin LA, Hepler PK (1996) Cytokinesis in higher plants. Cell 84: 812–824
Staiger CJ, Lloyd CW (1991) The plant cytoskeleton. Curr Opin Cell Biol 3: 33–42
van Lammeren AAM (1988) Structure and function of the microtubular cytoskeleton during endosperm development in wheat: an immunofluorescence study. Protoplasma 146: 18–27
Vantard M, Levilliers N, Hill A-M, Adoutte A, Lambert A-M (1990) Incorporation ofParamecium axonemal tubulin into higher plant cells reveals functional sites of microtubule assembly. Proc Natl Acad Sci USA 87: 8825–8829
Wick SM (1990) Spatial aspects of cytokinesis in plant cells. Curr Opin Cell Biol 3: 252–260
— (1991) The preprophase band. In: Lloyd CW (ed) The cytoskeletal basis of plant growth and form. Academic Press, London, pp 231–244
—, Seagull RW, Osborn M, Weber K, Gunning BES (1981) Immunofluorescence microscopy of organized microtubule arrays in structurally stabilized meristematic plant cells. J Cell Biol 89: 685–690
Zhang DH, Wadsworth P, Hepler PK (1990) Microtubule dynamics in living plant cells: confocal imaging of microinjected fluorescent brain tubulin. Proc Natl Acad Sci USA 87: 8820–8824
— — — (1993) Dynamics of microfilaments are similar, but distinct from microtubules during cytokinesis in living, dividing plant cells. Cell Motil Cytoskeleton 24: 151–155
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brown, R.C., Lemmon, B.E. Transition from mitotic apparatus to cytokinetic apparatus in pollen mitosis of the slipper orchid. Protoplasma 198, 43–52 (1997). https://doi.org/10.1007/BF01282130
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01282130