Summary
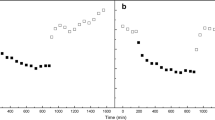
Cells ofChara corallina grown under high CO2 culture conditions were able to utilize exogenous HCO3 − to give appreciable rates of net photosynthesis. Since these rates of photosynthesis could be detected within 10 min of being transferred from high-CO2 to normal HCO3 − (pH 8.2) culture conditions, it would appear that the HCO3 −-accumulating system ofChara is not fully repressed under these high CO2 culture conditions. The membrane potential of these cells also responded to light/dark treatments in a manner consistent with the operation of a HCO3 − acquisition system. With prolonged exposure (2–6 days) to CPW/B, net photosynthesis continued to increase towards the expected control rate and, in parallel, the electrical responses elicited by light/dark treatments converged towards those obtained on control (CPW/B-grown)Chara cells. Charasomes were absent in CPW/CO2-grownChara, but redeveloped in mature cells once the culture was returned to CPW/B conditions; a minimum period of 7 days in CPW/B was required before charasomes were detected in tissue examined in the transmission electron microscope. As the above-detailed physiological and electrophysiological features were observed with both axial and whorl cells ofChara in which charasomes were completely absent, we conclude that this specialized organelle is not an essential component for photosynthetic utilization of exogenous HCO3 − in this species.
Similar content being viewed by others
Abbreviations
- CPW/B:
-
Chara pond water containing 1.0 mM NaHCO3, pH8.2
- CPW/CO2 :
-
Chara pond water containing dissolved CO2, pH 5.5
- DIC:
-
dissolved in organic carbon
- D.H.:
-
dark-induced membrane hyperpolarization
- L.H.:
-
light-induced membrane hyperpolarization
- TEM:
-
transmission electron microscopy
References
Brechignac F, Lucas WJ (1987) Photorespiration and internal CO2 accumulation inChara corallina as inferred from the influence of DIC and O2 on photosynthesis. Plant Physiol 83: 163–169
Coleman JR, Green LS, Berry JA, Togasaki RK, Grossman AR (1985) Adaptation ofChlamydomonas reinhardtii to air levels of CO2 and the induction of carbonic anhydrase activity. In: Lucas WJ, Berry JA (eds) Inorganic carbon uptake in aquatic photosynthetic organisms. American Society of Plant Physiologists, Rockville, MD, pp 339–359
Ferrier JM (1980) Apparent bicarbonate uptake and possible plasmalemma proton efflux inChara corallina. Plant Physiol 66: 1198–1199
Fisahn J, McConnaughey T, Lucas WJ (1989) Oscillations in extracellular current, external pH and plasma membrane properties in the alkaline bandsNitella andChara. J Exp Bot, submitted
Franceschi VR, Lucas WJ (1980) Structure and possible function(s) of charasomes; complex plasmalemma-cell wall elaborations present in some characean species. Protoplasma 104: 253–271
— — (1981) The glycosome ofChara: ultrastructure, development, and composition. J Ultrastruct Res 75: 218–228
— — (1982) The relationship of the charasome to chloride uptake inChara corallina: physiological and histochemical investigations. Planta 154: 525–537
Kaplan A (1985) Adaptation to CO2 levels: Induction and the mechanism for inorganic carbon uptake. In: Lucas WJ, Berry JA (eds) Inorganic carbon uptake in aquatic photosynthetic organisms. American Society of Plant Physiologists, Rockville, MD, pp 325–338
Keifer DW, Lucas WJ (1982) Potassium channels inChara corallina: control and interaction with the electrogenic H+ pump. Plant Physiol 69: 781–788
—, Spanswick RM (1978) Activity of the electrogenic pump inChara corallina as inferred from measurements of the membrane potential, conductance and potassium permeability. Plant Physiol 62: 653–661
Lucas WJ (1982) Mechanism of acquisition of exogenous bicarbonate by internodal cells ofChara corallina. Planta 156: 181–192
— (1983) Photosynthetic assimilation of exogenous HCO3 − by aquatic plants. Ann Rev Plant Physiol 34: 71–104
— (1985) Bicarbonate utilization byChara: a reanalysis. In: Lucas WJ, Berry JA (eds) Inorganic carbon uptake by aquatic photosynthetic organisms. American Society of Plant Physiologists, Rockville, MD, pp 223–254
—, Berry JA (1985) Inorganic carbon transport in aquatic photosynthetic organisms. Physiol Plant 65: 536–543
—, Franceschi VR (1981) Characean charasome complex and plasmalemma vesicle development. Protoplasma 107: 255–267
—, Shimmen T (1981) Intracellular perfusion and cell centrifugation studies on plasmalemma transport processes inChara corallina. J Membrane Biol 58: 227–237
—, Keifer DW, Pesacreta TC (1986) Influence of culture medium pH on charasome development and chloride transport inChara corallina. Protoplasma 130: 5–11
— —, Sanders D (1983) Bicarbonate transport inChara corallina: evidence for cotransport of HCO3 − with H+. J Membrane Biol 73: 263–274
Marcus Y, Volokita M, Kaplan A (1984) The location of the transporting system for inorganic carbon and the nature of the form translocated inChlamydomonas reinhardtii. J Exp Bot 35: 1136–1144
Miyachi S, Tsuzuki M, Yagawa Y (1985) Carbonic anhydrase in various microalgae. In: Lucas WJ, Berry JA (eds) Inorganic carbon uptake in aquatic photosynthetic organisms. American Society of Plant Physiologists, Rockville, MD, pp 145–154
Moroney JV, Husic HD, Tolbert NE (1985) Effect of carbonic anhydrase inhibitors on inorganic carbon accumulation byChlamydomonas reinhardtii. Plant Physiol 79: 177–183
Nishizaki Y (1968) Light-induced changes of bioelectric potential inChara. Plant Cell Physiol 9: 377–387
Ogawa T, Omata T, Miyano A, Inoue Y (1985) Photosynthetic reactions involved in the CO2-concentrating mechanism in the cyanobacterium,Anacystis nidulans. In: Lucas WJ, Berry JA (eds) Inorganic carbon uptake in aquatic photosynthetic organisms. American Society of Plant Physiologists, Rockville, MD, pp 287–304
Österlind S (1951) Inorganic carbon sources of green algae. IV. Photoactivation of some factor necessary for bicarbonate assimilation. Physiol Plant 4: 514–527
Pesacreta TC, Lucas WJ (1984) Plasma membrane coat and a coated vesicle-associated reticulum of membranes: their structure and possible interrelationship inChara corallina. J Cell Biol 98: 1537–1545
Price GD, Badger MR (1985) Inhibition by proton buffers of photosynthetic utilization of bicarbonate inChara corallina. Aust J Plant Physiol 12: 257–267
—, Whitecross MI (1983) Cytochemical localization of ATPase activity on the plasmalemma ofChara corallina. Protoplasma 116: 65–74
—, Badger MR, Bassett ME, Whitecross MI (1985) Involvement of plasmalemmasomes and carbonic anhydrase in photosynthetic utilization of bicarbonate inChara corallina. Aust J Plant Physiol 12: 241–256
Steemann Nielsen E (1960) Uptake of CO2 by the plants. In: Ruhland W (ed) Encyclopedia of plant physiology, vol 5, the assimilation of carbon dioxide. Springer, Berlin Heidelberg Göttingen, pp 70–84
Tsuzuki M, Miyachi S, Berry JA (1985) Intracellular accumulation of inorgainc carbon and its active species taken up byChlorella vulgaris 11 h. In: Lucas WJ, Berry JA (eds) Inorganic carbon uptake by aquatic photosynthetic organisms. American Society of Plant Physiologists, Rockville, MD, pp 53–66
Walker NA, (1980) The transport systems of charophyte and chlorophyte giant algae and their integration into modes of behavior. In: Spanswick RM, Lucas WJ, Dainty J (eds) Plant membrane transport: current conceptual issues. Elsevier/North Holland, Amsterdam New York Oxford, pp 287–300
—, Smith FA, Cathers IR (1980) Bicarbonate assimilation by freshwater charophytes and higher plants: I. membrane transport of bicarbonate ions is not proven. J Membrane Biol 57: 51–58
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lucas, W.J., Brechignac, F., Mimura, T. et al. Charasomes are not essential for photosynthetic utilization of exogenous HCO3 − inChara corallina . Protoplasma 151, 106–114 (1989). https://doi.org/10.1007/BF01403447
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01403447