Summary
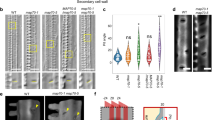
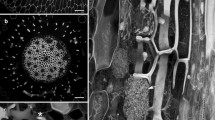
The epidermal transfer cells in developingVicia faba L. cotyledons are highly polarized. Extensive wall ingrowths occur on their outer periclinal walls and extend part way down both anticlinal walls. This ingrowth development serves to increase the surface area of the plasma membrane and thus maximize porter-dependent uptake of sugars from the seed apoplasm. In contrast, the inner periclinal walls of these transfer cells do not form wall ingrowths. We have commenced a study of the mechanisms responsible for establishing this polarity by first analysing the microtubule (MT) cytoskeleton in developing transfer cells. Thin sections of fixed cotyledons embedded in methacrylate resin were processed for immunofluorescence microscopy using monoclonal anti-β-tubulin and counterstained with Calcofluor White to visualize wall ingrowths. In epidermal cells of young cotyledons where wall ingrowths were yet to develop, MT labelling was detected around all cortical regions of the cell. However, in cells where wall ingrowths were clearly established, MT labelling was detected almost exclusively in cortical regions adjacent to the wall ingrowths. Little, if any, MT labelling was detected on the anticlinal or inner periclinal walls of these cells. This distribution of MTs was most prominent in cells with well developed wall ingrowths. In these cells, a subpopulation of MTs were also detected emanating from the subcortex and extending towards the wall ingrowth region. The possible role of MT distribution in establishing transfer cell polarity and wall ingrowth formation is discussed.
Similar content being viewed by others
Abbreviations
- MT:
-
microtubule
References
Baskin TI, Busby CH, Fowke LC, Sammut M, Gubler F (1992) Improvements in immunostaining samples embedded in methacrylate: localisation of microtubules and other antigens throughout developing organs in plants of diverse taxa. Planta 187: 405–413
Bonnemain JL, Bourquin S, Renault S, Offler C, Fisher DG (1991) Transfer cells: structure and physiology. In: Bonnemain JL, Delrot S, Lucas JW, Dainty J (eds) Recent advances in phloem transport and assimilate compartmentation. Ouest Editions, Nantes, pp 74–83
Brower DL, Hepler PK (1976) Microtubules and secondary wall deposition in xylem: the effects of isopropyln-phenylcarbamate. Protoplasma 87: 91–111
Cass DD, Karas I (1974) Ultrastructural organisation of the egg ofPlumbago zeylanica. Protoplasma 81: 49–62
Cleary AL, Hardham AR (1989) Microtubule organisation during development of stomatal complexes inLolium rigidum. Protoplasma 149: 67–81
Cyr RJ (1994) Microtubules in plant morphogenesis: role of the cortical array. Annu Rev Cell Biol 10: 153–180
Eleftheriou EP (1994) Abnormal structure of protophloem sieve-element cell wall in colchicine-treated roots ofTriticum aestivum L. Planta 193: 266–274
Falconer MM, Seagull RW (1986) Xylogenesis in tissue culture II: microtubules, cell shape and secondary wall patterns. Protoplasma 133: 140–148
Giddings TH, Staehelin A (1991) Microtubule-mediated control of microfibril deposition: a re-examination of the hypothesis. In: Lloyd CW (ed) The cytoskeletal basis of plant growth and form. Academic Press, London, pp 85–99
Gunning BES, Pate JS (1969) Transfer cells: plant cells with wall ingrowths, specialised in relation to short distance transport of solutes: their occurrence, structure and development. Protoplasma 68: 107–133
Harrington GN, Franceschi VR, Offler CE, Patrick JW, Tegeder M, Frommer WB, Harper JF, Hitz WD (1997) Cell specific expression of three genes involved in plasma membrane sucrose transport in developingVicia faba seed. Protoplasma 197: 160–173
Hogetsu T (1991) Mechanism for formation of the secondary wall thickening in tracheary elements: microtubules and microfibrils of tracheary elements ofPisum sativum L. andCommelina communis L. and the effects of amiprophosmethyl. Planta 185: 190–200
Huang B-Q, Russell SD, Strout GW, Mao L-J (1990) Organisation of isolated embryo sacs and eggs ofPlumbago zeylanica (Plumbaginaceae) before and after fertilisation. Am J Bot 77: 1401–1410
Johansson M, Walles B (1994) Functional anatomy of the ovule in broad bean (Viciafaba L.): ultrastructural seed development and nutrient pathways. Ann Bot 74: 233–244
Jones MGK, Northcote DH (1972) Nematode-induced syncytium: a multi-nucleate transfer cell. J Cell Sci 10: 789–809
Jung G, Wernicke W (1990) Cell shaping and microtubules in developing mesophyll of wheat (Triticum aestivum L.). Protoplasma 153: 141–148
Kobayashi H, Fukuda H, Shibaoka H (1988) Interrelation between the spatial disposition of actin filaments and microtubules during the differentiation of tracheary elements in culturedZinnia cells. Protoplasma 143: 29–37
Kropf DL (1992) Establishment and expression of cellular polarity in fucoid zygotes. Microbiol Rev 56: 316–339
Marc J, Mineyuki Y, Palevitz BA (1989) The generation and consolidation of a radial array of cortical microtubules in developing guard cells ofAllium cepa L. Planta 179: 516–529
Mascarenhas J (1993) Molecular mechanisms of pollen tube growth and differentiation. Plant Cell 5: 1303–1314
McDonald R, Fieuw S, Patrick JW (1996a) Sugar uptake by the dermal transfer cells of developing cotyledons ofVicia faba L.: experimental systems and general transport properties. Planta 198: 54–63
— — — (1996b) Sugar uptake by the dermal transfer cells of developing cotyledons ofVicia faba L.: mechanism of energy coupling. Planta 198: 502–509
—, Wang HL, Patrick JW, Offler CE (1995) The cellular pathway of sucrose transport in developing cotyledons ofVicia faba L. andPhaseolus vulgaris L.: a physiological assessment. Planta 196: 659–667
Meagher RB, Williamson RE (1994) The plant cytoskeleton. In: Meyerovitz EM, Somerville CR (eds)Arabidopsis. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 1049–1084
Offler CE, Patrick JW (1993) Pathway of photosynthate transfer in the developing seed ofVicia faba L.: a structural assessment of the role of transfer cells in unloading from the seed coat. J Exp Bot 44: 711–724
—, Liet E, Sutton EG (1997) Transfer cell induction in cotyledons ofVicia faba L. Protoplasma 200: 51–64
—, Nerlich SM, Patrick JW (1989) Pathway of photosynthate transfer in the developing seed ofVicia faba L.: transfer in relation to seed anatomy. J Exp Bot 40: 769–780
Palevitz BA, Hepler PK (1976) Cellulose microfibril orientation and cell shaping in developing guard cells ofAllium: the role of microtubules and ion accumulation. Planta 132: 71–93
Schnepf E, Pross E (1976) Differentiation and redifferentiation of a transfer cell: development of septal nectaries ofAloe andGasteria. Protoplasma 89: 105–115
Steer MW, Steer JM (1989) Pollen tube tip growth. New Phytol 111: 323–358
Wang HL, Offler CE, Patrick JW (1994) Nucellar projection transfer cells in the developing wheat grain. Protoplasma 182: 39–52
Wang X-D, Harrington GN, Patrick JW, Offler CE, Fieuw S (1995) Cellular pathway of photosynthate transport in coats of developing seed ofVicia faba L. andPhaseolus vulgaris L.: II. principal cellular site(s) of efflux. J Exp Bot 46: 49–64
Webb MC, Gunning BES (1994) Embryo sac development inArabidopsis thaliana II.: the cytoskeleton during megametagenesis. Sex Plant Reprod 7: 153–163
Willemse MTM, Van Lammeren AAM (1988) Structure and function of the microtubular cytoskeleton during megasporogenesis and embryo sac development inGasteria verrucosa (Mill) H. Duval. Sex Plant Reprod 1: 74–82
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bulbert, M.W., Offler, C.E. & McCurdy, D.W. Polarized microtubule deposition coincides with wall ingrowth formation in transfer cells ofVicia faba L. cotyledons. Protoplasma 201, 8–16 (1998). https://doi.org/10.1007/BF01280706
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01280706