Summary
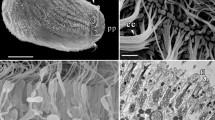
Allogromia sp. is a benthic foraminiferan protozoan which extends and withdraws a dynamic network of branching and anastomosing pseudopodia,i.e., reticulopods. Each reticulopod contains an elongate cytoskeleton composed primarily of microtubules (MT). When withdrawal was induced with artificial seawater supplemented with MgCl2, we found a time-dependent decrease in the number of reticulopodial MTs and a concomitant increase in 5-nm-diameter helical filaments. During the initial stages of withdrawal these helical filaments associated laterally to form loose aggregates. Later they formed dense paracrystalline aggregates, which appeared similar to those seen in the cell bodies of untreatedAllogromia juveniles prior to network extension. Similar results were obtained when withdrawal was induced by using seawater supplemented with other salts (NaCl, KCl). Treatment with an isotonic seawater substitute with an altered Na+:K+ ratio induced a momentary withdrawal, after which the organism recovered and reextended a network. During the withdrawal phase of this response, MTs became less abundant and aggregates of helical filaments more conspicuous. Together with earlier observations these findings suggest that helical filaments and paracrystalline material are an alternative or intermediate assembly form of MT proteins.
Similar content being viewed by others
References
Bowser, S. S., 1983: Fully correlative LM and HVEM analysis of motile fibrils and particles in the reticulopods ofAllogromia sp. (Protozoa, Foraminiferida). In: Proceedings of the 41st Annual Meeting of the Electron Microscopy Society of America (Bailey, G. W., ed.). San Francisco: San Francisco Press, Inc.
—,McGee-Russell, S. M., Rieder, C. L., 1984 a: Multiple fission inAllogromia sp., strain NF (Foraminiferida): Release, dispersal and ultrastructure of offspring. J. Protozool.28, 151–157.
—,Israel, H. A., McGee-Russell, S. M., Rieder, C. L., 1984 b: Surface transport properties of reticulopodia: Do intracellular and extracellular motility share a common mechanism? Cell Biol. Int. Rep.8, 1051–1063.
Curry, A., Butler, R. D., 1976: The ultrastructure, function and morphogenesis of the tentacle inDiscophrya sp. (Suctorida)Cileatea. J. Ultrastruct. Res.56, 164–176.
Fujiwara, K., Tilney, L. G., 1975: Substructural analysis of the microtubule and its polymorphic forms. Ann. N. Y. Acad. Sci.253, 27–50.
Hackney, M., Butler, R., 1981: Electrically induced tentacle retraction in the suctorian protozoanDiscophrya collini (Root). J. Protozool.28, 151–157.
Hauser, M., Schwab, D., 1974: Microtubules and helical microfilaments in the cytoplasm of the foraminiferAllogromia laticollaris Arnold. Investigations with vinblastine and deuterium oxide to demonstrate a close relationship. Cytobiologie9, 263–279.
Koury, S. T., 1982 a:In vivo microtubule assembly/disassembly states in the reticulopodia ofAllogromia sp., pp. 128–129. In: Proceedings of the 40th Annual Meeting of the Electron Microscopy Society of America (Bailey, G. W., ed.). Baton Rouge: Claitor's Pub. Div.
—, 1982 b: The ultrastructure ofAllogromia sp. during feeding steady state conditions and during induced retraction of the reticulopodial network. M.Sc. Thesis, State University of New York, Albany.
—,Bowser, S. S., McGee-Russell, S. M., 1981: Ultrastructural observations on induced retraction of the reticulopodial network ofAllogromia sp., pp. 604–605. In: Proceedings of the 39th Annual Meeting of the Electron Microscopy Society of America (Bailey, G. W., ed.). Baton Rouge: Claitor's Pub. Div.
Krishnan, N., Locke, M., 1971: Hot alcoholic phosphotungstic acid and uranyl acetate as routine stains for thick and thin sections. J. Cell Biol.50, 550–557.
Lee, J. J., Pierce, S., 1963: Growth and physiology of foraminifera in the laboratory: Part 4—monoxenic culture of an allogromiid with notes on its morphology. J. Protozool.10, 404–411.
McGee-Russell, S. M., 1974: Dynamic activities and labile microtubules in cytoplasmic transport in the marine foraminiferanAllogromia. Symp. Soc. exp. Biol.28, 157–189.
—,Allen, R. D., 1971: Reversible stabilization of labile microtubules in the reticulopodial network ofAllogromia. Adv. Cell mol. Biol.1, 153–184.
—,Trautwein, R., 1977: Low calcium transmembrane inhibition of the fixation-induced contractility system ofAllogromia. Ultrastructural correlates including crossbridges. Abstract 60. In: Abstracts of papers read at the fifth international congress on protozoology (Hutner, S. H., ed.). Society of Protozoologists, Lawrence, Kansas.
Molé-Bajer, J., Bajer, A., 1969: Studies of selected endosperm cells with the light and electron microscope: the technique. Cellule67, 257–265.
Murphy, D., Tilney, L., 1974: The role of microtubules in the movement of pigment granules in teleost melanophores. J. Cell Biol.61, 757–779.
Roth, L. E., Shigenaka, Y., 1970: Microtubules in the heliozoan axopodium. II. Rapid degradation by cupric and nickelous ions. J. Ultrastruct. Res.31, 356–374.
Schwab-Stey, H., Schwab, D., 1979: The transformation of cytoplasmic microtubules into helicies and paracrystals by halothane in the foraminiferAllogromia laticollaris Arnold. Z. mikr.-anat. Forsch.93, 751–762.
Shigenaka, Y., Roth, L. E., Pihlaja, D. J., 1971: Microtubules in the heliozoan axopodium. III. Degradation and reformation after dilute urea treatment. J. Cell Sci.8, 127–151.
Spurr, A., 1969: A low viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res.26, 31–43.
Tilney, L. G., Porter, K. R., 1967: Studies on the microtubules in heliozoa. II. The effect of low temperature on these structure in the formation and maintenance of the axopodia. J. Cell Biol.34, 327–343.
Tilney, L. G., 1968: Studies on the microtubules in heliozoa. IV. The effect of colchicine on the formation and maintenance of the axopodia and the re-development of pattern inActinosphaerium nucleofilum (Barrett). J. Cell Sci.3, 549–562.
Travis, J. L., Allen, R. D., 1981: Studies on the motility of the foraminifera. I. Ultrastructure of the reticulopodial network ofAllogromia laticollaris (Arnold). J. Cell Biol.90, 211–221.
Venable, J., Coggeshall, R., 1965: A simplified lead citrate stain for use in electron microscopy. J. Cell Biol.25, 407.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Koury, S.T., Bowser, S.S. & McGee-Russell, S.M. Ultrastructural changes during reticulopod withdrawal in the foraminiferan protozoanAllogromia sp., strain NF. Protoplasma 129, 149–156 (1985). https://doi.org/10.1007/BF01279912
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01279912