Abstract
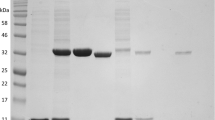
Catechol 2,3-dioxygenase from the thermophilic Bacillus thermoleovorans A2 was purified and characterized. The catechol 2,3-dioxygenase has a molecular mass of 135 000 Da and consists of four identical subunits of 34 700 Da. One iron per enzyme subunit was detected using atom absorption spectroscopy. Enzyme activity was not inhibited by EDTA, suggesting that the iron is tightly bound. Addition of hydrogen peroxide to the enzyme completely destroyed activity, indicating that the iron was in the divalent state. The isoelectric point of the enzyme was 4.8. The enzyme displayed optimal activity at pH 7.2 and 70°C. The half-life of the catechol 2,3-dioxygenase at the optimum temperature was 1.5 min under aerobic conditions and 10 min in a nitrogen atmosphere. This stability of the enzyme is comparable to the stability of the enzyme from the mesophilic Pseudomonas putida mt-2. The stability of the cloned enzyme in E. coli extracts was identical to the stability in wild-type extracts, suggesting that no stabilizing factors were present in Bacillus thermoleovorans A2 In whole cells the half-life of the enzyme at 70°C was approximately 26 min, when protein synthesis was disrupted by chloramphenicol; however, the activity remained constant when protein synthesis was not inhibited. From these results we concluded that catechol 2,3-dioxygenase from Bacillus thermoleovorans A2 is not particularly thermostable, but that the organism retains the ability to degrade phenol at high temperatures because of continuous production of this enzyme.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: October 10, 1998 / Accepted: March 18, 1999
Rights and permissions
About this article
Cite this article
Milo, R., Duffner, F. & Müller, R. Catechol 2,3-dioxygenase from the thermophilic, phenol-degrading Bacillus thermoleovorans strain A2 has unexpected low thermal stability. Extremophiles 3, 185–190 (1999). https://doi.org/10.1007/s007920050115
Issue Date:
DOI: https://doi.org/10.1007/s007920050115