Abstract
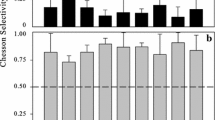
Grazing rates of mixed cultures of freshwater, heterotrophic nanoflagellates on two populations of bacterial prey present together at varying concentrations were measured by using fluorescently labeled bacteria. The effect of one population on the ingestion kinetics of the other was consistent with a theory based on competitive inhibition of enzymatic reactions. However, allochthonous bacteria, when present in low concentrations within a much larger population of small autochthonous bacteria, may be preferentially grazed, which is due to their large size.
Similar content being viewed by others
References
Anderson A, Larsson U, Hagström A (1986) Size selective grazing by a microflagellate on pelagic bacteria. Mar Ecol Prog Ser 33:51–57
Billen G, Servais P, Becquevort S (1990) Dynamics of bacterioplankton in oligotrophic and eutrophic aquatic environments: bottom-up or top-down control? Hydrobiologia 207:37–42
Billen G, Servais P, Fontigny A (1988) Growth and mortality in bacterial populations dynamics of aquatic environments. Arch Hydrobiol Beih Ergebn Limnol 31:173–183
Chrzanowski TH, Šimek K (1990) Prey-size selection by freshwater flagellated protozoa. Limnol Oceanogr 35:1429–1436
Edler L (1979) Recommendations for marine biological studies in the Baltic sea. Phytoplankton and chlorophyll. Baltic Mar Biologists Publ 5:1–38
Fenchel T (1982a) Ecology of heterotrophic microflagellate. I. Some important forms and their functional morphology. Mar Ecol Prog Ser 8:211–223
Fenchel T (1982) Ecology of heterotrophic microflagellate. II. Bioenergetics and growth. Mar Ecol Prog Ser 8:225–231
Fenchel T (1986) Protozoan filter feeding. Progr Protistol 1:65–113
Garcia-Lara J, Menon P, Servais P, Billen G (1991) Mortality of fecal bacteria in seawater. Appl Environ Microbiol 57:885–888
Garnier J, Billen G, Servais P (1992) Physiological characteristics and ecological role of small and large sized bacteria in a polluted river (Seine river, France). Arch Hydrobiol Ergbn Limnol 37:83–94
Garnier J, Servais P, Billen G (1992) Dynamics of bacterioplankton in the river Seine (France): impact of the Parisian effluents. Can J Microbiol 38:56–64
Goldman JC, Dennet MR (1990) Dynamics of prey selection by an omnivorous flagellate. Mar Ecol Prog Ser 59:183–194
Gonzales JM, Iriberri J, Egea L, Barcina I (1990) Differential rates of digestion of bacteria by freshwater and marine phagotrophic protozoa. Appl Environ Microbiol 56:1851–1857
Gonzales JM, Iriberri J, Egea L, Barcina I (1992) Characterization of culturability, protistan grazing and death of enteric bacteria in aquatic ecosystems. Appl Environ Microbiol 58:998–1004
Gonzales JM, Sherr EB, BF Sherr (1990) Size-selective grazing on bacteria by natural assemblages of estuarine flagellate and ciliates. Appl Environ Microbiol 56:583–589.
Grover JP (1990) Grazing by a heterotrophic microflagellate on two diatoms: functional and numerical responses in laboratory cultures. Arch Hydrobiol 119:197–213
McManus GB, Fuhrman JA (1988) Control of marine bacterioplankton populations: measurement and significance of grazing. Hydrobiologia 159:51–62
Monger BC, Landry MR (1990) Direct-interception feeding by marine zooflagellate: the importance of surface and hydrodynamic forces. Mar Ecol Prog Ser 65:123–140
Monger BC, Landry MR (1991) Prey-size dependency of grazing by free-living marine flagellate. Mar Ecol Prog Ser 74:239–248
Murdoch WW Oaten A (1975) Predation and population stability. Adv Ecol Res 9:1–131
Pace ML (1988) Bacterial mortality and the fate of bacterial production. Hydrobiologia 159:41–49
Porter KJ, Feig YS (1980) Use of DAPI for identifying and counting aquatic microflore. Limnol Oceanogr 25:943–948
Sanders RW Caron DA, Berningen UG (1992) Relationships between bacteria and heterotrophic nanoplankton in marine and fresh waters: an inter-ecosystem comparison. Mar Ecol Progr Ser 86:1–14
Sanders RW Porter KG, Bennett SJ, De Biase AE (1989) Seasonal patterns of bacterivory by flagellates, ciliates, rotifers, and cladocerans in a freshwater planktonic community. Limnol Oceanogr 34:673–687
Servais P, Billen G, Martinez J, Vives-Rego J (1989) Estimating bacterial mortality by the disappearance of 3H-labelled intracellular DNA. Technical validation and field measurements. FEMS Microbiol Ecol 62:119–126
Servais P, Billen G, Vives-Rego J (1985) Rate of bacterial mortality in aquatic environments. Appl Environ Microbiol 49:1448–1454
Servais P, Menon P (1991) Fate of autochthonous and fecal bacteria in marine ecosystems. Kieler Meeresforsch 8:290–296
Servais P, Vives-Rego J, Billen G (1992) Survival and mortality of bacteria in natural environments. In: Fry JC, Day MJ (eds) Release of genetically engineered and other microorganisms. Cambridge University Press, New York, pp 100–119
Sherr BF, Sherr EB, Fallon RD (1987) Use of monodispersed fluorescently bacteria to estimate in situ protozoan bacterivory. Appl Environ Microbiol 53:958–965
Sherr FB, Sherr EB, Pedros-Alio C (1989) Simultaneous measurement of bacterioplankton production and protozoan bacterivory in estuarine water. Mar Ecol Prog Ser 54:209–219
Sherr BF, Sherr EB, Rassoulzadegan F (1988) Rates of digestion of bacteria by marine phagotrophic protozoa: temperature dependence. Appl Environ Microbiol 54:1091–1095
Šimek K, Chrzanowski TH (1992) Direct and indirect evidence of size-selective grazing on pelagic bacteria by freshwater nanoflagellate. Appl Environ Microbiol 58:3715–3720
Simon M, Azam F (1989) Protein content and protein synthesis rates of planktonic marine bacteria. Mar Ecol Prog Ser 51:201–213
Watson SW, Novitsky TJ, Quinby HL, Valois FW (1977) Determination of bacterial number and biomass in the marine environment. Appl Environ Microbiol 33:940–946
Author information
Authors and Affiliations
Additional information
Correspondence to: P Servais
Rights and permissions
About this article
Cite this article
Menon, P., Becquevort, S., Billen, G. et al. Kinetics of flagellate grazing in the presence of two types of bacterial prey. Microb Ecol 31, 89–101 (1996). https://doi.org/10.1007/BF00175078
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00175078