Abstract
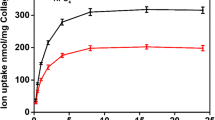
Factors that influence the rate and extent of the phenomenon in which calcium and phosphate ions become firmly associated with collagen-containing fibers prepared from beef tendon by two different extraction methods have been investigated. The fibers produced by both methods specifically require phosphate for calcium uptake and calcium is required for phosphate uptake. Ion uptake by both types is inhibited by Mg++, pyrophosphate, and an acidic peptide isolated from human serum. Whereas the collagen-containing fibers prepared by both methods induce ion uptake at nearly identical rates, only one of the methods produced a matrix that gives a positive response to the silver nitrate staining technique of von Kossa. Since both criteria of calcification are similarly influenced by reaction conditions and inhibitors, it is concluded that both are manifestations of different stages of the overall calcification process and that studies of ion uptake provide a quantitative assessment of calcification which could be of importance for investigating the mechanism and control of tissue mineralization.
Résumé
Les facteurs, influençant la vitesse et l'intensité du phénomène d'association des ions calcium et phosphates avec des fibres contenant du collagène, et préparés à partir du tendon de boeuf par deux méthodes d'extraction différentes, ont été étudiés. Les fibres, obtenues par ces deux méthodes, nécessitent spécifiquement du phosphate pour absorber du calcium et vice versa. L'absorption ionique des deux préparations est inhibée par du Mg++, du pyrophosphate et un peptide acidique, isolé du sérum humain. Alors que les fibres contenant du collagène, préparées selon les deux méthodes, présentent une absorption ionique à des vitesses sensiblement identiques, seule une des méthodes donne une matrice réagissant positivement à la technique de coloration au nitrate d'argent de von Kossa. Etant donné que les deux critères de calcification sont intéressés de façon identique par des conditions de réaction et par des inhibiteurs, il apparait que les deux facteurs sont des manifestations de différents stades de calcification et que des études d'absorption ionique fournissent une base quantitative d'appréciation de la calcification, pouvant être d'importance pour l'étude du mécanisme et de contrôle de la minéralisation tissulaire.
Zusammenfassung
Überprüft wurden die Faktoren, welche Geschwindigkeit und Ausmaß der Erscheinung beeinflussen, wobei Calcium- und Phosphationen sich mit den kollagenhaltigen, durch zwei verschiedene Extraktionsmethoden aus Rindersehnen gewonnenen Fasern eng zusammenbinden.
Die mit beiden Methoden zubereiteten Fasern benötigen spezifisch Phosphat für die Calciumaufnahme und Calcium für die Phosphataufnahme. Die Ionenaufnahme beider Arten wird durch Mg++, Pyrophosphat und saure, aus dem menschlichen Serum isolierte Peptide gehemmt. Während die nach beiden Methoden präparierten kollagenhaltigen Fasern eine Ionenaufnahme von beinahe gleicher Geschwindigkeit verursachen, ergibt nur eine dieser Methoden eine Matrix, die mit der Silbernitratfärbung nach vonKossa positiv reagiert. Da beide Calcifikationskriterien gleicherweise durch Reaktionsbedingungen und Inhibitoren beeinflußt werden, wird daraus geschlossen, daß beide Erscheinungen verschiedener Stadien des Gesamtcalcifikationsprozesses sind. Untersuchungen über die Ionenaufnahme ergeben eine quantitative Angabe der Verkalkung, welche für die Erforschung des Mechanismus und der Kontrolle der Mineralisation der Gewebe wichtig sein könnte.
Similar content being viewed by others
References
Bird, E. D., andW. C. Thomas, Jr.: The effect of various metals on mineralizationin vitro. Proc. Soc. exp. Biol (N.Y.)112, (440–643 (1963).
Boyd, E. S. andW. F. Neuman: The surface chemistry of bone — Ion binding properties of cartilage. J. biol. Chem.193, 243–251 (1951).
Einbinder, J. andM. Schubert: Binding of mucopolysaccharides and dyes by collagen. J. biol. Chem.188, 335–341 (1951).
Fiske, C. H., andY. SubbaRow: The colorimetric determination of phosphorus. J. biol. Chem.66, 375–380 (1925).
Fleisch, H., andS. Bisaz: Isolation from urine of pyrophosphate, a calcification inhibitor. Amer. J. Physiol.203, 671–675 (1962).
Glimcher, M. J.: Molecular biology of mineralized tissues with particular reference to bone. In: Biophysical science — A study program (J. L. Ondey, ed.), p. 359–393. The American Physical Society 1959.
—: Specificity of the molecular structure of organic matrices. In: Calcification in biological systems (R. F. Sognnaes, ed.). Amer. Ass. for Adv. Sci., Washington, D.C. 1960.
Gustavson, K. H.: The chemistry, and reactivity of collagen. New York: Academic Press 1956.
Hirschman, A., andA. Sobel: Composition of the mineral deposited duringin vitro calcification to the fluid phase. Arch. Biochem.110, 237–243 (1965).
Howard, J. E., W. C. Thomas, Jr., L. M. Barker, L. H. Smith, andC. L. Wadkins. The recognition and isolation from urine and serum of a peptide inhibitor to calcification. Johns Hopk. med. J.120, 119–136 (1967).
Johnson, L. C.: Morphologic analysis in pathology: The kinetics of disease and general biology of bone. In: Bone biodynamics (H. M. Frost, ed.), p. 543. Boston, Mass.: Little Brown & Co. 1964.
MacLean, F. C., andA. M. Budy: Radiation, isotopes and bone. New York: Academic Press 1964.
Miller, E. V., G. R. Martin, K. A. Piez, andM. I. Powers: Characterization of chick bone collagen and compositional changes associated with maturation. J. biol. Chem.242, 5481–5489 (1967).
Neuman, W. F., andC. C. Solomons: On the mechanisms of calcification — the remineralization of dentin. J. biol. Chem.235, 2502–2506 (1960).
Posner, A. S.: Relationship between diet and bone mineral ultrastructure. Fed. Proc.26, 1717–1722 (1967).
Robinson, R. A., andH. Sheldon: Crystal-collagen relationships in healing rickets. In: Calcification in biological systems (R. F. Sognnaes, ed.). Washington, D.C.: Amer. Ass. for Adv. Sci. 1958.
Sobel, A. E., andA. Hanok: Calcification: Reversible inactivation of calcification in vitro and related studies. J. biol. Chem.197, 669–685 (1952).
Thomas, W. C., Jr., andA. Tomita: Mineralization of human and bovine tissuesin vitro. Amer. J. Path.51, 621–628 (1967).
Urist, M. R., M. J. Moss, andJ. M. Adams: Calcification of tendon. Arch. Path.77, 594–608 (1964).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wadkins, C.L. Experimental factors that influence collagen calcificationin vitro . Calc. Tis Res. 2, 214–228 (1968). https://doi.org/10.1007/BF02279209
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02279209