Abstract
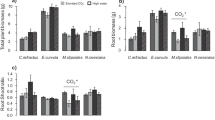
Recently, it has been suggested that small pots may reduce or eliminate plant responses to enriched CO2 atmospheres due to root restriction. While smaller pot volumes provide less physical space available for root growth, they also provide less nutrients. Reduced nutrient availability alone may reduce growth enhancement under elevated CO2. To investigate the relative importance of limited physical rooting space separate from and in conjunction with soil nutrients, we grew plants at ambient and double-ambient CO2 levels in growth containers of varied volume, shape, nutrient concentration, and total nutrient content. Two species (Abutilon theophrasti, a C3 dicot with a deep tap root andSetaria faberii, a C4 monocot with a shallow diffuse root system) were selected for their contrasting physiology and root architecture. Shoot demography was determined weekly and biomass was determined after eight and ten weeks of growth. Increasing total nutrients, either by increasing nutrient concentration or by increasing pot size, increased plant growth. Further, increasing pot size while maintaining equal total nutrients per pot resulted in increased total biomass for both species. CO2-induced growth and reproductive yield enhancements were greatest in pots with high nutrient concentrations, regardless of total nutrient content or pot size, and were also mediated by the shape of the pot. CO2-induced growth and reproductive yield enhancements were unaffected by pot size (growth) or were greater in small pots (reproductive yield), regardless of total nutrient content, contrary to predictions based on earlier studies. These results suggest that several aspects of growth conditions within pots may influence the CO2 responses of plants; pot size, pot shape, the concentration and total amount of nutrient additions to pots may lead to over-or underestimates of the CO2 responses of real-world plants.
Similar content being viewed by others
References
Arp WJ (1991) Effects of source-sink relations on photosynthetic acclimation to elevated CO2. Plant Cell Environ 14:869–875
Arp WJ, Drake BG (1991) Increased photosynthetic capacity ofScirpus olneyi after 4 years of exposure to elevated CO2. Plant Cell Environ 14:1003–1006
Barber SA (1984) Soil nutrient bioavailability: a mechanistic approach. Wiley, New York, New York, USA
Bazzaz FA (1990) Response of natural ecosystems to the rising CO2 levels. Ann Rev Ecol Syst 21:167–196
Bazzaz FA, Fajer ED (1992) Plant life in a CO2-rich world. Sci Amer 266:68–74
Boden TA, Sepanski RJ, Stoss FW (1992) Trends '91: A Compendium of Data on Global Change-Highlights. ORNL/CDIAC-049. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, Oak Ridge, Tennessee, USA
Carmi A, Hesketh JD, Enos WT, Peters DB (1983) Interrelationships between shoot growth and photosynthesis, as affected by root growth restriction. Photosynthetica 17:240–245
Cure JD, Acock B (1986) Crop responses to carbon dioxide doubling: a literature survey. Agric For Met 38:127–145
Drake BG, Leadley PW (1991) Canopy photosynthesis of crops and native plant communities exposed to long-term elevated CO2. Plant Cell Environ 14:853–860
Fajer ED, Bowers MD, Bazzaz FA (1992) The effect of nutrients and enriched CO2 environments on production of carbon-based allelochemicals inPlantago: a test of the carbon/nutrient balance hypothesis. Am Nat 140:707–723
Grulke NE, Riechers GH, Oechel WC, Hjelm U, Jaeger C (1990) Carbon balance in tussock tundra under ambient and elevated atmospheric CO2. Oecologia 83:485–494
Gurevitch J, Chester ST (1986) Analysis of repeated measures experiments. Ecology 67:251–255
Hilbert DW, Prudhomme TI, Oechel WC (1987) Response of tussock tundra to elevated carbon dioxide regimes: analysis of ecosystem CO2 flux through nonlinear modelling. Oecologia 72:466–472
Hogan KP, Smith AP, Ziska LH (1991) Potential effects of elevated CO2 and changes in temperature on tropical plants. Plant Cell Environ 14:763–778
Hunt R, Hand DW, Hannah MA, Neal AM (1991) Response to CO2 enrichment in 27 herbaceous species. Funct Ecol 5:410–421
Idso SB (1984) The case for carbon dioxide. J Env Sci 27:19–22
Idso SB (1986) Industrial age leading to the greening of the Earth? Nature 320:22
Idso SB (1991) Comment on “Modelling the seasonal contribution of a CO2 fertilization effect of the terrestrial vegetation to the amplitude increase in atmospheric CO2 at Mauna Loa Observatory” by G.H. Kohlmaier et al. Tellus 43B:338–341
Idso SB, Kimball BA (1991) Downward regulation of photosynthesis and growth at high CO2 levels. No evidence for either phenomenon in three-year study of sour orange trees. Plant Physiol 96:990–992
Jarvis PG (1989) Atmospheric carbon dioxide and forests. Philos Trans R Soc London B324:369–392
Joyner SP (ed) (1985) SAS/STAT guide for personal computers. Version 6 edition. SAS Institute, Cary, North Carolina, USA
Kimball BA (1986) CO2 stimulation of growth and yield under environmental constraints. In: Enoch HZ, Kimball BA (eds) Carbon dioxide enrichment of greenhouse crops. (Volume II; Physiology, yield and economics) CRC Press, Boca Raton, Florida, USA, pp 53–67
Kramer PJ (1981) Carbon dioxide concentration, photosynthesis, and dry matter production. BioScience 31:29–33
Krizek DT, Carmi A, Mirecki RM, Snyder FW, Bunce JA (1985) Comparative effects of soil moisture stress and restricted root zone volume on morphogenetic and physiological responses of soybean [Glycine max (L.) Merr]. J Exp Bot 36:25–38
Lawlor DW, Mitchell RAC (1991) The effects of increasing CO2 on crop photosynthesis and productivity: a review of field studies. Plant Cell Environ 14:807–818
McConnaughay KDM, Bazzaz FA (1991) Physical space as a soil resource. Ecology 72:94–103
McConnaughay KDM, Bazzaz FA (1992) The occupation and fragmentation of space: consequences of neighbouring roots. Funct Ecol 6:704–710
Norby RJ, Gunderson CA, Wullschleger SD, O'Neill EG, McCracken MK (1992) Productivity and compensatory responses of yellow-poplar trees in elevated CO2. Nature 357:322–324
Parish JAD, Bazzaz FA (1976) Underground niche separation in successional plants. Ecology 57: 1281–1288
Reekie EG, Bazzaz FA (1991) Phenology and growth in four annual species grown in ambient and elevated CO2. Can J Bot 69:2475–2481
Robbins NS, Pharr DM (1988) Effect of restricted root growth on carbohydrate metabolism and whole plant growth ofCucumis sativus L. Plant Physiol 87:409–413
Sionit N, Patterson DT (1984) Responses of C4 grasses to atmospheric CO2 enrichment. I. Effect of irradiance. Oecologia 65:30–34
Sokal RR, Rohlf FJ (1981) Biometry, second edition. W.H. Freeman and Company, New York, New York, USA
Stitt M (1991) Rising CO2 levels and their potential significance for carbon flow in photosynthetic cells. Plant Cell Environ 14:741–762
Strain BR, Bazzaz FA (1983) Terrestrial plant communities. In: Lemmon ER (ed) CO2 and Plants: The Response of Plants to Rising Levels of Atmospheric Carbon Dioxide. Westview, Boulder, Colorado, USA, pp 177–222
Thomas RB, Strain BR (1991) Root restriction as a factor in photosynthetic acclimation of cotton seedlings grown in elevated carbon dioxide. Plant Physiol 96:627–634
Velleman PF (ed) (1989) Data Desk® Statistics Guide, Volume II. Data Description, Inc., Northbrook, Illinois, USA
Warncke DD (1990) Testing artificial growth media and interpreting the results. In: Westerman RL (ed) Soil testing and plant analysis, 3rd edition. Soil Science Society of America, Madison, Wisconsin, USA, pp 337–357
Wieland NK, Bazzaz FA (1975) Physiological ecology of three codominant successional annuals. Ecology 56:681–688
Woodward FI, Thompson GB, McKee IF (1991) The effects of elevated concentrations of carbon dioxide on individual plants, populations, communities and ecosystems. Ann Bot 67 [Suppl] 1: 23–38
Wong SC (1979) Elevated atmospheric partial pressure of CO2 and plant growth. I. Interactions of nitrogen and photosynthetic capacity in C3 and C4 plants. Oecologia 44:68–74
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McConnaughay, K.D.M., Berntson, G.M. & Bazzaz, F.A. Limitations to CO2-induced growth enhancement in pot studies. Oecologia 94, 550–557 (1993). https://doi.org/10.1007/BF00566971
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00566971