Abstract
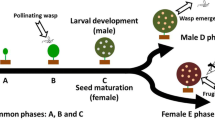
Fig trees and their pollinating wasps form ca. 750 pairs of obligate mutualists, mainly in the tropics. Survival of each partner depends on that of its associated species. Here, we examine the possible outcome of such an interaction at small population size. Using phenology data collected on Ficus natalensis in Gabon, we modelled wasp survival and the reproductive success of the trees according to the duration of receptivity of the tree, the amplitude of flowering seasonality, and the size of the fig tree population. Since the duration of receptivity is critical in these population level models, we also determined the influence of individual selection on this phenological trait. The models give three major results: (1) The minimum fig population size required to sustain a wasp population increases with the amplitude of seasonality, and decreases with increasing duration of receptivity; (2) tree population reproductive success is higher when the duration of receptivity is longer and when the population is large, but (3) individual selection toward a long duration of receptivity is weak or absent.
Similar content being viewed by others
References
Allee WC (1949) Group survival value for Philodina roseola, a rotifer. Ecology 30:395–397
Berg CC (1989) Classification and distribution of Ficus. Experientia 45:605–611
Bouček Z (1988) Australasian Chalcidoidea. CAB International, London
Bronstein JL (1989) A mutualism at the edge of its range. Experientia 45:622–636
Bronstein JL (1994) Our current understanding of mutualism. Q Rev Biol 69:31–51
Bronstein JL, Hossaert-McKey M (1995) Hurricane Andrew and a Florida fig pollination mutualism: resilience of an obligate interaction. Biotropica (in press)
Bronstein JL, Patel A (1992) Causes and consequences of withintree phenological patterns in the Florida strangling fig, Ficus aurea (Moraceae). Am J Bot 79:41–48
Bronstein JL, Gouyon PH, Gliddon C, Kjellberg F, Michaloud G (1990) Ecological consequences of flowering asynchrony in monoecious figs: a simulation study. Ecology 71:2145–2156
Compton SG (1993) One way to be a fig. Afr Entomol 1:151–152
Compton SG, Ross SJ, Thornton IWB (1994) Pollinator limitation of fig tree reproduction on the island of Anak Krakatau (Indonesia). Biotrpica 26:180–186
Corlett RT (1984) The phenology of F. benjamina and F. microcarpa in Singapore. J Singapore Acad Sci 13:30–31
Douglas AE, Smith DC (1989) Are endosymbioses mutualistic? Trends Ecol Evol 4:350–352
Gautier-Hion A, Michaloud G (1989) Figs: are they keystone resources for frugivorous vertebrates throughout the tropics? A test in Gabon. Ecology 70:1826–1833
Hill DS (1967) Figs of Hong Kong. Hong Kong University Press, Hong Kong
Hossaert-McKey M, Gibernau M, Frey JE (1994) Chemosensory attraction of fig wasps to substances produced by receptive figs. Entomol Exp Appl 70:185–191
Janzen DH (1985) The natural history of mutualisms. In: Boucher DH (ed) The biology of mutualism, ecology and evolution. Croom Helm, London Sydney, pp 40–99
Khadari B, Gibernau M, Anstett MC, Kjellberg F, Hossaert-McKey M (1995) When figs are waiting for pollinators. The duration of female receptivity in two fig species (Ficus carica and F. aurea). Am J Bot (in press)
Kjellberg F, Maurice S (1989) Seasonality in the reproductive phenology of Ficus: its evolution and consequences. Experientia 45:653–660
Kjellberg F, Doumesche B, Bronstein JL (1988) Longevity of a fig wasp (Blastophaga psenes). Proc K Ned Akad Wet (C) 91:117–122
Kjellberg F, Gouyon PH, Ibrahim M, Raymond M, Valdeyron G (1987) The stability of the symbiosis between dioecious figs and their pollinators: a study of Ficus carica L. and Blastophaga psenes L. Evolution 41:693–704
Lambert FR, Marshall AG (1991) Keystone characteristics of birddispersed Ficus in a Malaysian lowland rain forest. J Ecol 79:793–809
Maynard Smith J (1982) Evolution and the theory of games. Cambridge University Press, Cambridge, UK
McKey D (1989) Population biology of figs: applications for conservation. Experientia 45:661–673
Michaloud G (1988) Aspects de la reproduction des figuiers monoïques en forêt équatoriale Africaine. Thèse Doctorale, Université des Sciences et Techniques du Languedoc, Montpellier, France
Milton K, Windsor DM, Morrison DW, Estribi MA (1982) Fruiting phenology of two neotropical Ficus species. Ecology 63:752–762
Morrison DW (1978) Foraging ecology and energetics of the frugivory bat Artibeus jamaicensis. Ecology 59:716–723
Nason JD, Herre EA, Hamrick JL (1996) Paternity analysis of the breeding structure of strangler fig populations: evidence for substantial long-distance wasp dispersal. Jr Biogeogr (in press)
Newton LE, Lomo A (1979) The pollination of Ficus vogelii in Ghana. Bot J Linn Soc 78:21–30
Norstog K, Fawcett PKS, Vovides AP (1992) Beetle pollination of two species of Zamia: evolutionary and ecological considerations. Paleobotanist 41:149–158
Patel A, Anstett MC, Hossaert-McKey M, Kjellberg F (1995) Pollinators entering female dioecious figs: why commit suicide? J Evol Biol (in press)
Primack RB (1985) Longevity of individual flowers. Annu Rev Ecol Syst 16:15–37
Ramirez W (1970) Host specificity of fig wasps (Agaonidae). Evolution 24:681–691
Smith CM (1994) Seasonality and synchrony: a site comparison of reproductive phenology in three neotropical figs. Master's thesis, University of Arizona, Tucson
Terborgh J (1986) Keystone plant resources in the tropical rain forest. In: Soulé ME (ed) Conservation biology, the science of scarcity and diversity. Sinauer, Sunderland, Mass, pp 330–344
Wiebes JT (1979) Co-evolution of figs and their insect pollinators. Annu Rev Ecol Syst 10:1–12
Windsor DM, Morrison DW, Estribi MA, de Leon B (1989) Phenology of fruit and leaf production by “strangler” figs on Barro Colorado Island, Panama. Experientia 45:647–652
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Anstett, MC., Michaloud, G. & Kjellberg, F. Critical population size for fig/wasp mutualism in a seasonal environment: effect and evolution of the duration of female receptivity. Oecologia 103, 453–461 (1995). https://doi.org/10.1007/BF00328683
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00328683