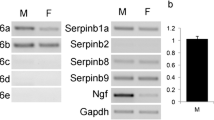
Abstract
Antibodies against 10 different secretory proteins from the accessory sex glands of the male rat were used for immunohistochemical studies of salivary and lacrimal glands from intact and castrated rats, at the light- and electron-microscopic levels. In the parotid gland, secretory acinar cells showed immunoreactivity with antibodies against prostatic binding protein, cystatin-related peptide and acid phosphatase (isoenzyme pI 8.0; 5.6) typical of ventral prostate, and seminal vesicle secretion VI. Western blotting analysis indicated that immunoreactivity against prostatic binding protein was attributable to a subunit, presumably C3. Acid phosphatase pI 5.6 showed a molecular weight of 66 kDa, which is at variance with the prostatic form. Immunoreactivity for secretory transglutaminase, derived from the coagulating gland, was restricted to myoepithelial and stromal cells. In castrated animals, the immunoreactivity of acinar cells was reduced to the background level, whereas stromal transglutaminase immunoreactivity was unaltered. The distribution pattern of immunoreactivity for the proteins mentioned was almost identical in the lacrimal gland. Significant differences were however observed in the immunoreactivity of the inframandibular gland, where serous glandular cells were non-immunoreactive for seminal proteins, with the exception of acid phosphatase isoenzyme pI 8.0. Granules present in the convoluted granular ducts were immunoreactive particularly for acid phosphatase (isoenzyme pI 5.6)but much less for cystatin-related peptide; immunoreactivity was reduced after castration. The straight portion of the inframandibular duct system was immunoreactive for transglutaminase, but no influence of castration was visible. The distribution of immunoreactivity for seminal proteins present in the salivary and lacrimal glands and the pronounced androgen-dependence of their expression point to functional relationships of the respective proteins at both glandular sites.
Similar content being viewed by others
References
Abrescia P, Guardiola J, Felsani A, Metafora S (1982) Expression in males and genomic organization of the gene(s) coding for a major protein secreted by the rat seminal vesicle epithelium. Nucleic Acids Res 10:159
Antakly T, Laperche Y, Feigelson P (1982) Synthesis and immunocytochemical localization of α2-microglobulin in the duct cells of the rat submaxillary gland. J Histochem Cytochem 30:1293–1296
Ashley PL, MacDonald RJ (1985) Tissue-specific expression of kallikrein-related genes in the rat. Biochemistry 24:4520–4527
Aumüller G, Seitz J (1990) Protein secretion and secretory processes in male accessory sex glands. Int Rev Cytol 121:127–230
Aumüller G, Seitz J, Heyns W, Flickinger CJ (1982) Intracellular localization of prostatic binding protein (PBP) in rat prostate by light and electron microscopic immunocytochemistry. Histochemistry 76:497–516
Barka T (1980) Biologically active polypeptides in submandibular glands. J Histochem Cytochem 28:836–859
Barka T, Gresik EW, Noen H van der (1985) Monoclonal antibodies against rat saliva and salivary gland antigens. J Histochem Cytochem 33:209–218
Baskin D, Erlandsen SL, Parsons JA (1979) Influence of hydrogen peroxide or alcoholic sodium peroxide on the immunocytochemical detection of growth hormone and prolactin after osmium fixation. J Histochem Cytochem 27:1290–1292
Berg T, Wassdal I, Sletten K (1992) Immunochemical localization of rat submandibular gland esterase B (homologous to the RSKG-7 kallikrein gene) in relation to other serine proteases of the kallikrein family. J Histochem Cytochem 40:83–92
Brady JM, Wines DR, MacDonald RJ (1989) Expression of two kallikrein gene family members in the rat prostate. Biochemistry 28:5203–5210
Caramia F (1966) Ultrastructure of mouse submaxillary gland. II. Effects of castration of the male. J Ultrastruct Res 16:524–542
Carmo-Fonseca M, Vaz Y (1989) Immunocytochemical localization and lectin-binding properties of the 22 kDa secretory protein from rat ventral prostate. Biol Reprod 40:153–164
Celis L, Claessens F, Peeters B, Heyns W, Verhoeven G, Rombauts W (1993) Proteins interacting with an androgen-responsive unit in the C3(1) gene intron. Mol Cell Endocrinol 94:165–172
Chamberlin LL, Mpanias OD, Wang TY (1983) Isolation, properties and androgen regulation of a 20-kilodalton protein from rat ventral prostate. Biochemistry 22:3072–3077
Cohen S (1960) Purification of a nerve growth-promoting protein from the mouse salivary gland and its neurocytotoxic antiserum. Proc Natl Acad Sci USA 46:302–311
Devos A, Clercq N de, Vercaeren I, Heyns W, Rombauts W, Peeters B (1993) Structure of rat genes encoding androgen-regulated cystatin-related proteins (CRPs): a new member of the cystatin superfamily. Gene 125:159–167
Fesüs L, Davies PJA, Piacentini M (1991) Apoptosis: molecular mechanisms in programmed cell death. Eur J Cell Biol 56:170–177
Forsgren B, Björk P, Carlström K, Gustafsson J-A, Pousette A, Högberg B (1979) Purification and distribution of a major protein in rat prostate that binds estramustine, a nitrogen mustard derivative of estradiol-17-β. Proc Natl Acad Sci USA 76:3149–3153
Girard LR, Castle AM, Hand AR, Castle JD, Mirels L (1993) Characterization of common salivary protein 1, a product of rat submandibular, sublingual, and parotid glands. J Biol Chem 268:26592–26601
Hazen-Martin DJ, Landreth G, Simson JA (1987) Immunocytochemical localization of nerve growth factor in mouse salivary glands. Histochem J 19:210–216
Helminen HJ, Ericsson JLE, Rytoluoto R, Vanha-Perttula T (1975) Acid phosphatases of the rat ventral prostate. In: Goland M (ed) Normal and abnormal growth of the prostate. Thomas, Springfield, Illinois, pp 275–316
Hemschoote K, Peeters B, Dirckx L, Claessens F, Clercq N de Heyns W, Winderickx J, Bannwart W, Rombauts W (1988) A single 12.5 kilobase androgen-regulated mRNA encoding multiple proline-rich polypeptides in the ventral prostate of the rat. J Biol Chem 263:19159–19165
Heyns W, Moor P de (1977) Prostatic binding protein. A steroid binding protein by rat ventral prostate. Eur J Biochem 78:221–230
Heyns W, Peeters B, Mous J, Rombauts W, Moor P de (1978) Purification and characterization of prostatic binding protein and its subunits. Eur J Biochem 89:181–186
Heyns W, Bossyns D,Peeters B, Rombauts W (1982) Study of the proline-rich polypeptide boundto prostatic binding protein in the rat ventral prostate. J Biol Chem 257:7407–7413
Higgins SJ, Burchell JM, Mainwaring JP (1976) Androgen-dependent synthesis of basic secretory proteins by the rat seminal vesicle. J Biochem 158:271
Ho KC, Snoek R, Quarmby V, Viskochil DH, Rennie PS, Wilson EM, French FS, Bruchovsky N (1989) Primary structure and androgen regulation of a 20-kilodalton protein specific to rat ventral prostate. Biochemistry 28:6367–6373
Ikejima T, Ito S (1984) Carbonic anhydrase in mouse salivary glands and saliva: a histochemical, immunohistochemical, and enzyme activity study. J Histochem Cytochem 32:625–635
Isemura S, Saitoh E, Ito S, Isemura M, Sanada K (1984) Cystatin S: a cysteine proteinase inhibitor of human saliva. J Biochem 96:1311–1314
Iversen JM, Kauffman DL, Keller PJ, Robinovitch M (1985) Isolation and partial characterization of two populations of secretory granules from rat parotid glands. Cell Tissue Res 240:441–447
Junqueira LCU, Toledo AMS, Saad A (1964) Studies on the physiology of rat and mouse submaxillary glands I and III. In: Sreebny LM, Meyer J (eds) Salivary glands and their secretion. Macmillan, New York, pp 354–369
Kim S-K (1984) Changes in the secretory acinar cells of the rat parotid gland during aging. Anat Rec 209:345–354
Kishimoto R, Gomi T, Izaike Y, Nagai K, Nakagawa H (1982) A novel nuclear protein in rat ventral prostate. Androgen-dependent and age-related change. Biochem Biophys Acta 718:165–171
Kivelä T (1992) Antigenic profile of the human lacrimal gland. J Histochem Cytochem 40:629–642
Lea OA, Petrusz P, French FS (1979) Prostatein, a major secretory protein of the rat ventral prostate. J Biol Chem 254:6196–6202
Luft JH (1961) Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol 9:409–414
Mezzetti G, Loor R, Liao S (1979) Androgen-sensitive sperminebinding proteins of rat ventral prostate. Purification of the protein and characterization of the hormonal effect. Biochem J 184:431–440
Morell JI, Gresik EW, Barka T (1987) Autoradiographic localization of dihydrotestosterone binding in the major salivary glands and other androgen-responsive organs of the mouse. J Histochem Cytochem 35:1053–1058
Ørstavik TB, Carretero OA, Hayashi H, Scicli GA, Johansen L (1982) Immunohistochemical localization of tonin and its relation to kallikrein in rat salivary glands. J Histochem Cytochem 30:1123–1129
Oliver C, Dromy R, Hart TK (1989) Density gradient separation of two populations of lysosomes from rat parotid acinar cells. J Histochem Cytochem 37:1645–1652
Ostrowski MC, Kistler MK, Kistler WS (1979) Purification and cell free synthesis of a major protein from rat seminal vesicle secretion. A potential marker for androgen action. J Biol Chem 254:383–390
Paradis G, Dube JY, Chapdelaine P, Tremblay RR (1987) In vitro translation of human prostatic acid phosphatase mRNA and processing of the translation products by microsomal membranes and endoglycosidase. J Biochem Cell Biol 65:921–924
Parker MG, Scrace GT, Mainwaring WIP (1978) Testosterone regulates the synthesis of major proteins in rat ventral prostate. Biochem J 170:115–121
Parkkila S, Kaunisto K, Rajaniemi L, Kumpulainen T, Jokinen K, Rajaniemi H (1990) Immunohistochemical localization of carbonic anhydrase isoenzymes VI, II, and I in human parotid and submandibular glands. J Histochem Cytochem 38:941–947
Rins de David ML, Caceres A, Goldraj A (1990) Contribución al estudio del dimorfismo sexual en la glándula submandibular de la rata. Acta Odontol Latinoam 5:63–69
Rins de David ML, Finkelberg de Sterin A, Goldraj A (1991) Influence of gonadic hormones on the rat submaxillary gland. Arch Int Physiol Biochim Biophys 99:107–109
Roiko K, Jänne OA, Vihko P (1990) Primary structure of rat secretory acid phosphatase and comparison to other acid phosphatases. Gene 89:223–229
Rytöluoto-Kärkkäinen R, Jauhiainen A, Vanha-Perttula T (1984) Comparison of acid phosphatases in the rat prostatic complex and seminal vesicles. J Urol 132:814–820
Seitz J (1985) Brochemie, Cytochemie and Immunhistochemie von sauren Phosphatasen.Habilitationsschrift, Fachbereich Medizin, Marburg
Seitz J, Aumüller G (1980) Cytochemistry and biochemistry of acid phosphatases I. Cytochemistry and isoelectric focusing of acid phosphatases of the rat ventral prostate. Histochemistry 67:99–111
Seitz J, Aumüller G (1989) Secretory proteins from male accessory sex glands: isolation, biochemical and functional characterization. In: Voigt KD, Holstein AE, Graesslin D, (eds) Reproductive biology and medicine. Diesbach, Berlin, pp 112–118
Seitz J, Keppler C, Rausch U, Aumüller G (1990) Immunohistochemistry of secretory transglutaminase from rodent prostate. Histochemistry 93:525–530
Seitz J, Keppler C, Hüntemann SB (1991) Purification and characterization of transglutaminases from the genital tract of the male rat. J Chromatogr 587:55–60
Shaw PA, Cox JL, Barka T, Naito Y (1988) Cloning and sequencing of cDNA encoding a rat salivary cysteine proteinase inhibitor inducible by β-adrenergic agonists. J Biol Chem 263:18133–18137
Shiba A, Shiba KS, Suzuki K (1986) Analysis of salivary proteins by thin layer dodecylsulphate polyacrylamide gel electrophoresis. J Oral Rehabil 13:263–271
Simson JAV, Fenters R, Chao J (1983) Electron microscopic immunostaining of kallikrein in rat submandibular glands. J Histochem Cytochem 31:301–306
Simson JAV, Condon J, Fenters R, Chao L, Chao J (1988) Immunocytochemical localization of a kallikrein-like serine protease (esterase A) in rat salivary glands. Anat Rec 221:475–481
Steinhoff M, Eicheler W, Holterhus PM, Rausch U, Seitz J, Aumüller G (1994) Hormonally induced changes in apocrine secretion of transglutaminase in the rat dorsal prostate and coagulating gland. Eur J Cell Biol 65:49–59
Sternberger LA, Hardy PH, Cuculis JJ, Meyer HG (1970) The unlabeled antibody enzyme method of immunohistochemistry. J Histochem Cytochem 18:315–333
Tenniswood MP, Bird C, Clark AF (1976) Acid phosphatases: androgen dependent markers of rat prostate. Can J Biochem 54:350–357
Terracio L, Rule A, Salvato J, Douglas WHJ (1985) Immunofluorescent localization of an androgen-dependent isoenzyme of prostatic acid phosphatase in rat ventral prostate. Anat Rec 213:131–139
Towbin H, Staehelin T, Gordon J (1976) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Vihko P, Kurkela R, Porvari K, Herrala A, Lindfors A, Lindquist Y, Schneider G (1993) Rat acid phosphatase: overexpression of active, secreted enzyme by recombinant Baculovirus-infected insect cells, molecular properties and crystallization. Proc Natl Soc Acad Sci USA 90:799–803
Vercaeren I, Winderickx J, Devos A, Peeters B, Heyns W (1992) An effect of androgens on the length of the poly(A)-tail and alternative splicing cause size heterogeneity of the messenger ribonucleic acids encoding cystatin-related protein. Endocrinology 131:2496–2502
Williams-Ashman HG (1984) Transglutaminases and the clotting of mammalian seminal fluids. Mol Cell Biochem 58:51–61
Winderickx J, Swinnen K, Dijck P van, Verhoeven G, Heyns W (1989) Kallikrein-related protease in the rat ventral prostate: cDNA cloning and androgen regulation. Mol Cell Endocrinol 62:217–226
Winderickx J, Hemschoote K, Clercq N de, Dijck P van, Peeters B, Rombauts W, Verhoeven G, Heyns W (1990) Tissue-specific expression and androgen regulation of different genes encoding rat prostatic 22-kilodalton glycoproteins homologous to human and rat cystatin. Mol Endocrinol 4:657–667
Winderickx J, Vercaeren I, Verhoeven G, Heyns W (1994) Androgen-dependent expression of cystatin-related protein (CRP) in the exorbital lacrimal gland of the rat. J Steroid Biochem Mol Biol 48:165–170
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Aumüller, G., Arce, E.A., Heyns, W. et al. Immunocytochemical localization of seminal proteins in salivary and lacrimal glands of the rat. Cell Tissue Res 280, 171–181 (1995). https://doi.org/10.1007/BF00304522
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00304522