Abstract
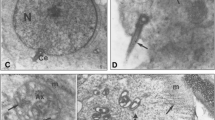
The objective of this study was to determine the cellular and subcellular distribution of small nuclear ribonucleoprotein particles (snRNPs) in the adult rat testis in relation to the different cell types at the various stages of the cycle of the seminiferous epithelium. The distribution of snRNPs in the nucleus and cytoplasm of germ cells was quantitated in an attempt to correlate RNA processing with morphological and functional changes occurring during the development of these cells. Light-microscopic immunoperoxidase staining of rat testes with polyclonal anti-Sm and monoclonal anti-Y12 antibodies localized spliceosome snRNPs in the nuclei and cytoplasm of germ cells up to step 10 spermatids. Nuclear staining was intense in Sertoli cells, spermatogonia, spermatocytes, and in the early steps of round spermatid development. Although comparatively weaker, cytoplasmic staining for snRNPs was strongest in mid and late pachytene spermatocytes and early round spermatids. Quantitative electron-microscopic immunogold labeling of Lowicryl embedded testicular sections confirmed the light-microscopic observations but additionally showed that the snRNP content peaked in the cytoplasm of midpachytene spermatocytes and in the nuclei of late pachytene spermatocytes. The immunogold label tended to aggregate into distinct loci over the nuclear chromatin. The chromatoid body of spermatids and spermatocytes and the finely granular material in the interstices of mitochondrial aggregates of spermatocytes were found to be additional sites of snRNP localization and were intensely labeled. This colocalization suggests that these dense cytoplasmic structures may be functionally related. Anti-U1 snRNP antibodies applied to frozen sections showed the same LM localization pattern as spliceosome snRNPs. Anti-U3 snRNP antibodies applied to frozen sections stained nucleoli of germ cells where pre-rRNA is spliced.
Similar content being viewed by others
References
Andrade LEC, Chan EKL, Raska I, Peebles CL, Roos G, Tan EM (1991) Human autoantibody to a novel protein of the nuclear coiled body: immunological characterization and cDNA cloning of p80-coilin. J Exp Med 173:1407–1419
Biggiogera M, Fakan S, Leser G, Martin TE, Gordon J (1990) Immunoelectron microscopical visualization of ribonucleoproteins in the chromatoid body of mouse spermatids. Molec Reprod Dev 26:150–158
Biggiogera M, Schack M-L von, Martin TE, Gordon J, Muller M, Fakan S (1993) Immunoelectron microscope localization of snRNP, hnRNP, and ribosomal proteins in mouse spermatogenesis. Molec Reprod Dev 35:261–271
Billings PB, Allen RW, Jensen FC, Hoch SO (1982) Anti-RNP monoclonal antibodies derived from a mouse strain with lupus-like autoimmunity. J Immunol 128:1175–1180
Carmo-Fonseca M, Pepperkok R, Carvalho MT, Lamond AI (1992) Transcription-dependent colocalization of the U1, U2, U4/U6, and U5 snRNPs in coiled bodies. J Cell Biol 117:1–14
Carter KC, Taneja KL, Lawrence JB (1991) Discrete nuclear domains of poly(A) RNA and their relationship to the functional organization of the nucleus. J Cell Biol 115:1191–1202
Daoust R, Clermont Y (1955) Distribution of nucleic acids in germ cells during the cycle of the seminiferous epithelium in the rat. Am J Anat 96:255–279
Eddy EM (1974) Fine structural observations on the form and distribution of nuage in germ cells of the rat. Anat Rec 178:731–758
Fakan S, Leser G, Martin TE (1984) Ultrastructural distribution of nuclear ribonucleoproteins as visualized by immunocytochemistry on thin sections. J Cell Biol 98:358–363
Fawcett DW, Eddy EM, Phillips DM (1970) Observation on the fine structure and relationships of the chromatoid body in mammalian spermatogenesis. Biol Reprod 2:129–153
Fu X-D, Maniatis T (1990) Factor required for mammalian spliceosome assembly is localized to discrete regions in the nucleus. Nature 343:437–441
Hecht NB (1987) Gene expression during spermatogenesis. Ann NY Acad Sci 513:90–101
Hecht NB, Liem H (1984) Mitochondrial DNA is synthesized during meiosis and early spermiogenesis in the mouse. Exp Cell Res 154:293–298
Heyderman H (1979) Immunoperoxidase technique in histopathology: applications, methods, and controls. J Clin Pathol 32:971–978
Huang S, Spector DL (1991) Nascent pre-mRNA transcripts are associated with nuclear regions enriched in splicing factors. Genes Dev 5:2288–2302
Iatrou KAW, Dixon GW (1977) Messenger RNA sequences in the developing trout testis. Cell 10:433–441
Kano Y, Komatsu H, Kankanoin K, Fujiwara Y (1978) Distribution of small molecular weight nuclear RNA in transcriptionally active and inactive avian cells. Exp Cell Res 115:444–447
Krainer AR, Maniatis T (1985) Multiple factors including the small nuclear ribonucleoproteins U1 and U2 are necessary for pre-RNA splicing in vitro. Cell 42:725–735
Krimer DB, Esponda P (1980) Presence of polysaccharides and proteins in the chromatoid body of mouse spermatids. Cell Biol Int Rep 4:265–270
Lamond AI (1991) Nuclear RNA processing. Curr Opin Cell Biol 1:519–525
Lamond AI, Carmo-Fonseca M (1993) The coiled body. Trends in Cell Biol 3:198–204
Leblond CP, Clermont Y (1952) Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann NY Acad Sci 55:548–573
Lerner MR, Boyle JA, Mount SM, Wolin SL, Steitz JA (1980) Are snRNPs involved in splicing? Nature 283:220–223
Lobo SM, Marzluff WF, Seufert AC, Dean WL, Schultz GA, Simerly C, Schatten G (1988) Localization and expression of U1 RNA in early mouse embryo development. Dev Biol 127:349–361
Maniatis T, Reed R (1987) The role of small nuclear ribonucle-oprotein particles in pre-mRNA splicing. Nature 325:673–678
Monesi V (1964) Ribonucleic acid synthesis during mitosis and meiosis in the mouse testis. J Cell Biol 22:521–532
Monesi V (1965) Synthetic activity during spermatogensis in the mouse. RNA and protein synthesis. Exp Cell Res 39:197–224
Moore GPM (1971) DNA-dependent RNA synthesis in fixed cells during spermiogenesis in the mouse. Exp Cell Res 68:462–465
Morales CR, Hecht NB (1993) Poly(A)+ ribonucleic acids are enriched in spermatocyte nuclei but not in chromatoid bodies in the rat testis. Biol Reprod 50:309–319
Morales CR, Oko R, Clermont Y (1994) Molecular cloning and developmental expression of an mRNA encoding the 27 kDa outer dense fiber protein of rat spermatozoa. Molec Reprod Dev 37:229–240
Oko R (1988) Comparative analysis of proteins from the fibrous sheath and outer dense fibers of rat spermatozoa. Biol Reprod 39:169–172
Oko R, Clermont Y (1990) Mammalian spermatozoa: structure and assembly of the tail. In: Gagnon C (ed) Controls of sperm motility: biological and clinical aspects. CRC Press, Boston, pp 3–27
Oko R, Clermont Y (1991) Biogenesis of specialized cytoskeletal elements of rat spermatozoa. NY Acad Sci 637:203–223
Parvinen M, Parvinen LM (1979) Active movements of the chromatoid body: a possible transport mechanism for haploid gene products. J Cell Biol 80:621–628
Prather R, Simerly C, Schatten G, Pilch DR, Lobo SM, Marzluff WF, Dean WL, Schultz GA (1990) U3 snRNPs and nucleolar development during oocyte maturation, fertilization and early embryogenesis in the mouse: U3 snRNA and snRNPs are not regulated coordinate with other snRNAs and snRNPs. Dev Biol 138:247–255
Puvion E, Viron A, Assens C, Leduc EH (1984) Immunocytochemical identification of nuclear structures containing snRNPs in isolated rat liver cells. J Ultrastruct Res 87:180–189
Reimer G, Pollard KM, Penning CA, Ochs RL, Lischwe MA, Busch H, Tan EM (1987) Monoclonal antibody from a (New Zealand black x New Zealand white) F1 mouse and some human scleroderma sera target an Mr 34,000 nucleolar protein of the U3 RNP particle. Arthritis and Rheumatism 30:793–800
Reuter R, Appel B, Bringmann P, Rinke J, Luhrman R (1984) 5′ terminal caps of snRNAs are reactive with antibodies specific for 2, 2, 7,-trimethylguanosine in whole cells and nuclear matrices. Exp Cell Res 154:548–560
Rogers J, Wall R (1980) A mechanism for RNA splicing. Proc Natl Acad Sci USA 7:1877–1879
Russell LD, Frank B (1978) Ultrastructural characterization of nuage in spermatocytes of the rat testis. Anat Rec 10:79–98
Saunders PTK, Millar SM, Maguire SM, Sharpe TM (1992) Stage specific expression of rat transition protein 2 mRNA and possible localization to the chromatoid body of step 7 spermatids by in situ hybridization using a nonradioactive riboprobe. Molec Reprod Dev 33:385–391
Schultz MC (1990) Three structures associated with the nucleolus in male rat germinal cells: round body, coiled body, and “nebecula” and general presence of round body at male meiosis. Am J Anat 189:11–23
Söderström K-O (1981) Labeling of the chromatoid body by [3H]uridine in rat pachytene spermatocytes. Exp Cell Res 131:489–491
Söderström K-O, Parvinen M (1976) Incorporation of [3H]uridine by the chromatoid body during rat spermatogenesis. J Cell Biol 70:239–246
Spector DL, Schrier WH, Busch H (1983) Immunoelectron microscopic localization of snRNPs. Biol Cell 49:1–10
Spector DL, Fu X-D, Maniatis T (1991) Associations between distinct pre-mRNA splicing components and the cell nucleus. EMBO J 10:3467–3481
Susi FR, Clermont Y (1970) Fine structural modifications of the rat chromatoid body during spermiogenesis. Am J Anat 129:177–192
Thorne-Tjomsland G, Clermont Y, Hermo L (1988) Contribution of Golgi apparatus components to the formation of the acrosomic system and chromatoid body in rat spermatids. Anat Rec 221:591–598
Walt H, Armbruster BL (1984) Actin and RNA are components of the chromatoid bodies in spermatids of the rat. Cell Tissue Res 236:487–490
Wang J, Cao L-G, Wang YL, Pederson T (1991) Localization of premessenger RNA at discrete nuclear sites. Proc Natl Acad Sci USA 88:7391–7395
Zeive GW, Sauterer RA (1990) Cell biology of the snRNP particles. Biochem Mol Biol 25:1–46
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Moussa, F., Oko, R. & Hermo, L. The immunolocalization of small nuclear ribonucleoprotein particles in testicular cells during the cycle of the seminiferous epithelium of the adult rat. Cell Tissue Res. 278, 363–378 (1994). https://doi.org/10.1007/BF00414179
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00414179