Summary
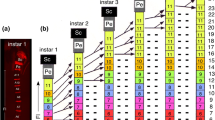
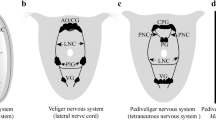
The development of the sensory neurons of the legs of the blowfly,Phormia regina has been described from the third instar larva to the late pupa using immunohistochemical staining. The leg discs of the third instar larva contain 8 neurons of which 5 come to lie in the fifth tarsomere of the developing leg. Whereas 2 neurons persist at least to the late pupa, the other cells degenerate. The first neurons of gustatory sensilla arise in the fifth tarsomere at about 1.5 h after formation of the puparium. Most of these sensilla, however, appear within a short time period beginning at about 18 h. The femoral chordotonal sensory neurons first appear at the time of formation of the puparium, as a mass of cells situated in the distal femur. During later pupal development 2 groups of these cells come to lie at the femur-trochanter border, where they become the proximal femoral chordotonal organ of the adult; the remaining cells become the distal femoral chordotonal organ. Other scolopidial neurons appear later in development. The nerve pathways of the late pupal leg are established either by the axons of the cells that are present in the larval leg disc or by new outgrowing processes of sensory neurons. In the tibia, the initial direction of new outgrowth differs in different regions of the segment: proximal tibial neurons grow distally, while distal tibial neurons grow initially proximally.
Similar content being viewed by others
References
Auerbach C (1936) The development of legs, wings and halteres in wild type and some mutant strains ofDrosophila melanogaster. Trans R Soc Edin 58:787–817
Bacon JP, Murphey RK (1984) Receptive fields of cricket giant interneurons are related to their dendritic structure. J Physiol 352:601–623
Bate CM (1976) Pioneer neurones in an insect embryo. Nature 260:54–56
Bowen ID (1981) Techniques for demonstrating cell death. In: Bowen ID, Lockshin RA (eds) Cell death in biology and pathology. Chapman and Hall, London, pp 379–444
Caudy M, Bentley D (1986) Pioneer growth cone steering along a series of neuronal and non-neuronal cues of different affinities. J Neurosci 6:1781–1795
Caudy M, Bentley D (1987) Pioneer growth cone behavior at a differentiating limb segment boundary in the grasshopper embryo. Dev Biol 119:454–465
Dambly-Chaudiere C, Ghysen A (1986) The sense organs in theDrosophila larva and their relation to the embryonic pattern of sensory neurons. Wilhelm Roux Arch 195:222–228
Debaisieux P (1938) Organes scolopidiaux des pattes d'insectes. La Cellule 47:77–202
Dethier VG (1976) The hungry fly. Harvard University Press, Cambridge, Mass
Dickinson MH, Palka J (1987) Physiological properties, time of development, and central projection are correlated in the wing mechanoreceptors ofDrosophila. J Neurosci 7:4201–4208
Ghysen A (1980) The projection of sensory neurons in the central nervous system ofDrosophila: Choice of the appropriate pathway. Dev Biol 78:521–541
Grabowski CT, Dethier VG (1954) The structure of the tarsal chemoreceptors of the blowfly,Phormia regina Meigen. J Morphol 94:1–17
Hansen K, Hansen-Delkeskamp E (1983) The development of taste and tactile hairs in the pharate flyProtophormia terraenovae (Diptera, Calliphoridae) and the embryonal cricketAcheta domestica (Orthopteroidea, Ensifera). Zoomorphology 102:241–259
Heathcote RD (1981) Differentiation of an identified sensory neuron (SR) and associated structures (CTO) in grasshopper embryos. J Comp Neurol 202:1–18
Hertweck H (1931) Anatomie und Variabilität des Nervensystems und der Sinnesorgane vonDrosophila melanogaster. Z Wiss Zool 139:560–664
Hill D, Bell VA, Chadwick LE (1947) Rearing of the blowfly,Phormia regina Meigen on a sterile synthetic diet. Ann Entomol Soc Am 40:213–216
Jan LY, Jan YN (1982) Antibodies to horseradish peroxidase as specific neuronal markers inDrosophila and in grasshopper embryos. Proc Natl Acad Sci USA 79:2700–2704
Jan YN, Ghysen A, Barbel CS, Jan LY (1985) Formation of neuronal pathways in the imaginal discs ofDrosophila melanogaster. J Neurosci 5:2453–2464
Keilin D (1915) Recherches sur les larves de diptères cycloraphes. Bull Sci Fr Belge Ser 7:15–198
Keshishian H, Bentley D (1983a) Embryogenesis of peripheral nerve pathways in grasshopper legs I. The initial nerve pathway to the CNS. Dev Biol 96:89–102
Keshishian H, Bentley D (1983b) Embryogenesis of peripheral nerve pathways in grasshopper legs III. Development without pioneer neurons. Dev Biol 96:116–124
Kramer JJ de, Molen LG van der (1984) Development of labellar taste hairs in the blowfly,Calliphora vicina (Insecta, Diptera). Zoomorphology 104:1–10
Kutsch W, Bentley D (1987) Programmed death of peripheral pioneer neurons in the grasshopper embryo. Dev Biol 123:517–525
Lawrence PA (1966) Development and determination of hairs and bristles in the milkweed bug,Oncopeltus fasciatus (Lygaeidae, Hemiptera). J Cell Sci 1:475–498
Lee JK, Altner H (1985) Sensory cell degeneration in the ontogeny of the chemosensitive sensilla in the labial palp-pit organ of the butterfly,Pieris rapae L. (Insecta, Lepidoptera). Cell Tissue Res 242:279–288
Murphey RK (1981) The structure and development of a somatotopic map in crickets: the cercal afferent projection. Dev Biol 88:236–246
Murphey RK, Possidente D, Pollack G, Merritt DJ (1989) Modality specific axonal projections in the CNS of the fliesPhormia andDrosophila. J Comp Neurol (in press)
Palka J (1987) Axon guidance in the insect periphery. Development 99:307–309
Palka J, Malone MA, Ellison RL, Wigston DJ (1986) Central projections of identifiedDrosophila sensory neurons in relation to their time of development. J Neurosci 6:1822–1830
Peters W (1965) Die Sinnesorgane an den Labellen vonCalliphora erythrocephala Mg. (Diptera). Z Morphol Ökol Tiere 55:259–320
Peterson BA, Weeks JC (1988) Somatotopic mapping of sensory neurons innervating mechanosensory hairs on the larval prolegs ofManduca sexta. J Comp Neurol 275:128–144
Possidente D, Murphey RK (1989) Genetic control of sexually dimorphic axon morphology inDrosophila sensory neurons. Dev Biol 132:448–457
Reichert H, Meier T (1988) Segmental homology of auditory and leg sensory systems in locusts. Soc Neurosci [Abstr] 14:377
Römer H, Marquart V, Hardt M (1988) Organization of a sensory neuropile in the auditory pathway of two groups of orthoptera. J Comp Neurol 275:201–215
Ruiten ThM van, Sprey ThE (1974) The ultrastructure of the developing leg disk ofCalliphora erythrocephala. Z Zellforsch 147:373–400
Sanes JR, Hildebrand JG (1976) Origin and morphogenesis of sensory neurons in an insect antenna. Dev Biol 51:300–319
Schubiger M, Palka J (1985) Genetic suppression of putative guidepost cells: effects on establishment of nerve pathways inDrosophila melanogaster. Dev Biol 108:399–410
Selzer R, Schaller-Selzer L (1987) Structure and function of luminal neurons in the early embryonic antenna of the american cockroachPeriplaneta americana. Dev Biol 122:363–373
Shiraishi A, Tanabe Y (1974) The proboscis extension response and tarsal and labellar chemosensory hairs in the blowfly. J Comp Physiol 92:161–179
Snow PM, Patel NH, Harrelson AL, Goodman CS (1987) Neural-specific carbohydrate moiety shared by many surface glycoproteins inDrosophila and grasshopper embryos. J Neurosci 7:4137–4144
Sprey ThE (1971) Cell death during development of the imaginal disks ofCalliphora erythrocephala. Neth J Zool 21:221–264
Spurr AR (1969) A low-viscosity epoxy embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Tomlinson A, Ready DF (1987) Neuronal differentiation in theDrosophila ommatidium. Dev Biol 120:366–376
Walthall WW, Murphey RK (1986) Positional information, compartments, and the cercal sensory system of crickets. Dev Biol 113:182–200
Weismann A (1864) Die nachembryonale Entwicklung der Musceiden nach Beobachtungen anMusca vomita undSarcophaga carnaria. Z Wiss Zool XIV:187–336
Yetman S, Pollack GS (1986) Central projections of labellar taste hairs in the blowfly,Phormia regina Meigen. Cell Tissue Res 245:555–561
Zdarek J, Fraenkel G (1972) The mechanism of puparium formation in flies. J Exp Zool 179:315–324
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lakes, R., Pollack, G.S. The development of the sensory organs of the legs in the blowfly,Phormia regina . Cell Tissue Res. 259, 93–103 (1990). https://doi.org/10.1007/BF00571434
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00571434