Summary
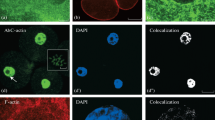
The distribution of actin in dividing endothelial cells of the rat cornea was studied by fluorescence microscopy by means of the nitrobenzoxadiazole conjugated derivative of the actin-binding toxin phallacidin (NBD-Ph). In normal noninjured tissue, fluorescence is limited to an area at or near the plasma membrane. Twenty-four hours after a corneal freeze injury, stress fibers are detected but only in those cells that are migrating into the wound area. By 48 h post-injury, cells in various stages of mitosis can be identified. During metaphase, anaphase, and telophase, diffuse cytoplasmic staining is observed, although the spindle region remains free of fluorescence. At various sites along the plasma membrane, fluorescence appears stronger compared to other regions. During the latter two stages of proliferation, NBD-Ph positive material can be seen within cell processes. In addition, a band of this material is observed within the region that corresponds to the cleavage furrow. As the daughter cells separate, actin can be detected within the cytoplasmic bridge. The results indicate that NBD-Ph can be used to study the distribution of actin in cells that were proliferating in vivo, and these patterns appear similar to those obtained with immunological methods on cultured cells.
Similar content being viewed by others
References
Aubin JE, Weber K, Osborn M (1979) Analysis of actin microfilament-associated proteins in the mitotic spindle and cleavage furrow of PtK2 cells by immunofluorescence microscopy. Exp Cell Res 124:93–109
Barak LS, Yocum RR, Nothnagel EA, Webb WW (1980) Fluorescence staining of the actin cytoskeleton in living cells with 7-nitrobenz-2-oxa-1,3 diazole phallacidin. Proc Natl Acad Sci USA 77:980–984
Barak LS, Yocum RR, Webb WW (1981a) In vivo staining of cytoskeletal actin by autointernalization of nontoxic concentrations of nitrobenzoxadiazole-phallacidin. J Cell Biol 89:368–372
Barak LS, Nothnagel EA, DeMarco EF, Webb WW (1981b) Differential staining of actin in metaphase spindles with 7-nitrobenz-2-oxa-1,3 diazole phallacidin and fluorescent DNase: Is actin involved in chromosomal movement? Proc Natl Acad Sci USA 78:3034–3038
Biberfeld G, Fagaeus A, Lenkei R (1974) Reaction of human smooth muscle antibody with thyroid cells. Clin Exp Immunol 18:371–377
Burnside B, Laties AM (1976) Actin filaments in apical projections of the primate pigmented epithelial cell. Invest Ophthalmol 15:570–575
Cande WZ, Lazarides E, McIntosh JR (1977) A comparison of the distribution of actin and tubulin in the mammalian mitotic spindle as seen by indirect immunofluorescence. J Cell Biol 72:552–567
Drenckhahn D, Gröschel-Stewart U (1977) Localization of myosin and actin in ocular nonmuscle cells. Immunofluorescence-microscopic, biochemical and electron-microscopic studies. Cell Tissue Res 181:493–503
Fagraeus A, The E, Biberfeld G (1973) Reaction of human smooth muscle antibody with thymus medullary cells. Nature New Biol 246:113–115
Farrow LJ, Holborow EJ, Brighton WD (1971) Reaction of human smooth muscle antibody with liver cells. Nature New Biol 232:186–187
Faure JP, Kim YZ, Graf B (1971) Formation of giant cells in the corneal endothelium during its regeneration after destruction by freezing. Exp Eye Res 12:6–12
Gawaldi N (1974) Characterization and distribution of microfilaments in dividing locust testis cells. Cytobios 10:17–35
Geiger B (1981) The association of rhodamine labelled α-actinin with actin bundles in demembranated cells. Cell Biol Int Rep 5:627–634
Gipson IK, Anderson RA (1977) Actin filaments in normal and migrating corneal epithelial cells. Invest Ophthalmol Vis Sci 16:161–166
Goldman RD, Lazarides E, Pollack R, Weber K (1975) The distribution of actin in non-muscle cells: the use of actin antibody in the localization of actin within the microfilament bundles of mouse 3T3 cells. Exp Cell Res 90:333–344
Gordon SR (1980) The cell biology of corneal endothelial wound repair. Ph.D. Thesis, University of Vermont, Burlington, Vermont
Gordon SR, Rothstein H (1978) Studies on corneal endothelial growth and repair. I. Microfluorometric and autoradiographic analysis of DNA synthesis, mitosis and amitosis following freeze injury. Metabol Ophthalmol 2:57–63
Gordon SR, Essner E, Rothstein H (1981) The in situ localization of actin in ocular tissues with 7-nitrobenz-2-oxa-1,3 diazole phallacidin. IRCS Med Sci 9:956–957
Gordon SR, Essner E, Rothstein H (1982) In situ demonstration of actin in normal and injured ocular tissues using 7-nitrobenz-2-oxa-1,3 diazole phallacidin. Cell Motility 2:343–354
Hayashi M, Ohnishi K, Hayashi K (1980) Dense precipitate of brain tubulin with skeletal muscle myosin. J Biochim 87:1347–1355
Herman IM, Pollard TD (1979) Comparison of purified anti-actin and fluorescent-heavy meromyosin staining patterns in dividing cells. J Cell Biol 80:509–520
Herman IM, Crisona NJ, Pollard TD (1981) Relation between cell activity and the distribution of cytoplasmic actin and myosin. J Cell Biol 90:84–91
Hirsch M, Faure JP, Marquet O, Payrau P (1975) Régénération del endothélium corneén chez le lapin: Étude microscopique et relation avec l'épaisseur de la corneén. Arch Ophthalmol (Paris) 35:269–278
Hitchcock SE (1977) Regulation of motility in non-muscle cells. J Cell Biol 74:1–15
Hynes RO, Destree AT (1978) Relationship between fibronectin (LETS protein) and actin. Cell 15:875–886
Kalnins VI, Subrahmanyan L, Gotlieb AI (1981) The reorganization of cytoskeletal fibre systems in spreading procine endothelial cells in culture. Eur J Cell Biol 24:36–44
Korn ED (1978) Biochemistry of actomyosin-dependent cell motility (A review). Proc Natl Acad Sci USA 75:588–599
LaFountain JR (1975) What moves chromosomes, microtubules or microfilaments? Biosystems 7:363–369
Lazarides E, Revel JP (1979) The molecular basis of cell movement. Sci Am 241:100–113
Lazarides E, Weber K (1974) Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci USA 71:2268–2272
Rahi A, Ashton N (1978) Contractile proteins in retinal endothelium and other non-muscle tissues of the eye. Brit J Ophthalmol 62:627–643
Raemeakers FCS, Osborn M, Schmid E, Weber K, Bloemendal H, Franke WW (1980) Indentification of the cytoskeleton proteins in lens-forming cells, a special epithelioid cell type. Exp Cell Res 127:309–327
Rothstein H, Gordon SR (1980) Studies on corneal endothelial growth and repair. II. Increased transcription as detected by incorporation of 3H-uridine and 3H-actinomycin D. Tissue Cell 12:647–659
Sanger JW (1975a) Changing patterns of actin localization during cell division. Proc Natl Acad Sci USA 72:1913–1916
Sanger JW (1975b) Presence of actin during chromosomal movement. Proc Natl Acad Sci USA 72:2451–2455
Schloss JA, Milsted A, Goldman RD (1977) Myosin subfragment binding for the localization of actin-like microfilaments in cultured cells. J Cell Biol 74:794–815
Schroeder TE (1973) Actin in dividing cells: contractile ring filaments bind heavy meromyosin. Proc Natl Acad Sci USA 70:1688–1692
Shimo-Oka T, Hayashi M, Watanabe Y (1980) Tubulin-myosin interaction. Some properties of binding between tubulin and myosin. Biochem 19:4921–4926
Singer II (1979) The fibronexus: a transmembrane association of fibronectin-containing fibers and bundles of 5 nm microfilaments in hamster and human fibroblasts. Cell 16:675–685
Weiland T (1977) Modifications of actins by phallotoxins. Naturwissenschaften 64:303–309
Willingham MC, Yamada SS, Davies PJA, Rutherford AV, Gallo MG, Pastan I (1981) Intracellular localization of actin in cultured fibroblasts by electron microscopic immunocytochemistry. J Histochem Cytochem 29:17–37
Author information
Authors and Affiliations
Additional information
Supported by NIH grant EY02711
Rights and permissions
About this article
Cite this article
Gordon, S.R. The localization of actin in dividing corneal endothelial cells demonstrated with nitrobenzoxadiazole phallacidin. Cell Tissue Res. 229, 533–539 (1983). https://doi.org/10.1007/BF00207696
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00207696