Summary
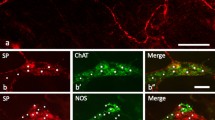
With an antiserum to the molluscan cardioactive tetrapeptide FMRF-amide immunoreactive perikarya and nerve fibers were identified in the central and peripheral nervous system of the pond snail Lymnaea stagnalis. Their localization is described. The same antiserum yielded reactive product in particular cells of the epithelium of the alimentary tract. The use of two different fixatives, glutaraldehyde, and a mixture of glutaraldehyde, picric acid, and acetic acid (GPA) showed that certain nerve cells can be identified only in material fixed with either the one or the other of these two fixatives, a result which indicates that in Lymnaea more than one FMRF-amide-like substance may occur.
“Positive” axon endings were found in the periphery of various nerves, i.e., in places where neurohormones are released into the blood. Other fibers were found to end, probably synaptically, on other neurons, on epithelial cells in the stomach, and between muscle cells in various parts of the body, e.g., in the heart. In these cases the FMRF-amide-like substance may function as a neurotransmitter or a neuromodulator.
Similar content being viewed by others
References
Agarwal RA, Ligon PJB, Greenberg MJ (1972) The distribution of cardioactive agents among molluscan species and tissues. Comp Gen Pharmac 3:249–260
Blanchi D, Noviello L, Libonati M (1973) A neurohormone of cephalopods with cardioexcitatory activity. Gen Comp Endocrinol 21:267–277
Boer HH, Schot LPC, Roubos EW, Maat A ter, Lodder JC, Reichelt D, Swaab DF (1979) ACTH-like immunoreactivity in two electrotonically coupled giant neurons in the pond snail Lymnaea stagnalis. Cell Tissue Res 202:231
Boer HH, Schot LPC, Veenstra JA, Reichelt D (1980) Immunocytochemical identification of neural elements in the central nervous system of a snail, some insects, a fish, and a mammal with an antiserum to the molluscan cardio-excitatory tetrapeptide FMRF-amide. Cell Tissue Res 213:21–27
Cottrell GA (1978) Actions of a “molluscan cardioexcitatory neuropeptide” on identified 5-hydroxy-tryptamine-containing neurons and their follower neurons in Helix aspersa. J Physiol (Lond) 284:130–131
Cottrell GA (1980) Voltage dependent and voltage independent actions of the molluscan neuropeptide Phe-Met-Arg-Phe-NH2 on a snail neurone. J Physiol (Lond) 300:41
Dockray GJ, Vaillant C, Williams RC (1981) New vertebrate brain-gut peptide related to a molluscan neuropeptide and a opioidpeptide. Nature 292:656–657
Elo JE (1938) Das Nervensystem von Lymnaea stagnalis (L.). Lam Ann Zool Vanamo 6:1–40
Frontali N, Williams L, Welsh JH (1967) Heart excitatory and inhibitory substances in molluscan ganglia. Comp Biochem Physiol 22:833–841
Geraerts WPM, Leeuwen JPThM van, Nuyt K, de With ND (1981) Cardioactive peptides of the CNS of the pulmonate snail Lymnaea stagnalis. Exp 37:1168–1169
Greenberg MJ, Price DA (1979) FMRF amide, a cardioexcitatory neuropeptide of molluscs: An agent in search of a mission. Am Zool 19:163–174
Greenberg MJ, Price DA (1980) Cardioregulatory peptides in molluscs. In: Bloom FE (ed) Peptides: Integrators of cell and tissue function. Raven Press, New York, pp 107–126
Grimmelikhuijzen CJP, Dockray GJ, Schot LPC (1982) FMRF amide-like immunoreactivity in the nervous system of Hydra. Histochemistry 73:499–509
Hekstra GP, Lever J (1960) Some effects of ganglion extirpation in Lymnaea stagnalis. Proc Kon Ned Akad Wet C 63:271–282
Lloyd PE (1978) Distribution and molecular characteristics of cardioactive peptides in the snail, Helix aspersa. J Comp Physiol 128:269–276
Pearse AGE (1969) The cytochemistry and ultrastructure of polypeptide hormone producing cells of the APUD-series and the embryologie, physiologic and pathologic implications of the concept. J Histochem Cytochem 17:303–313
Pearse AGE, Takor Takor T (1976) Neuroendocrine embryology and the APUD concept. Clin Endocrinol 5: Suppl: 229–244
Plesch B (1977) An ultrastructural study of the innervation of the musculature of the pond snail, Lymnaea stagnalis (L.) with reference to peripheral neurosecretion. Cell Tissue Res 183:353–369
Price DA, Greenberg MJ (1977) Purification and characterization of a cardio-excitatory neuropeptide from the central ganglia of a bivalve mollusc. Prep Biochem 7:50–62
Scharrer B (1978) Peptidergic neurons: facts and trends. Gen Comp Endocrinol 34:50–62
Schot LPC, Boer HH, Swaab DF, Noorden S van (1981) Immunocytochemical demonstration of peptidergic neurons in the central nervous system of the pond snail Lymnaea stagnalis with antisera raised to biologically active peptides of vertebrates. Cell Tissue Res 216:273–291
Smock T, Arch S, Lloyd P (1978) Secretory sources of egg-laying induction also influence the isolated Aplysia heart. Neurosci Abstr 4:206
Sternberger LA (1974) Immunocytochemistry foundation of immunology series (Oster A, Weiss L, eds). Prentice Hall Inc, Englewood Cliffs, New Jersey
Swaab DF, Pool CW (1975) Specificity of oxytocin and vasopressin immunofluorescence. J Endocrinol 66:263–272
Swindale NV, Benjamin (1976) The anatomy of neurosecretory neurones in the pond snail Lymnaea stagnalis (L.). Phil Trans Roy Soc Lond B 274:169–202
Wendelaar Bonga SE (1970) Ultrastructure and histochemistry of neurosecretory cells and neurohaemal areas in the pond snail Lymnaea stagnalis (L.). Z Zellforsch 108:190–224
Wydenes J, Minnen J, van Boer HH (1980) A comparative study on neurosecretion demonstrated by the alcian blue — alcian yellow technique in three terrestrial pulmonates (Styllommatophora). Cell Tissue Res 210:47–56
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schot, L.P.C., Boer, H.H. Immunocytochemical demonstration of peptidergic cells in the pond snail Lymnaea stagnalis with an antiserum to the molluscan cardioactive tetrapeptide FMRF-amide. Cell Tissue Res. 225, 347–354 (1982). https://doi.org/10.1007/BF00214687
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00214687