Summary
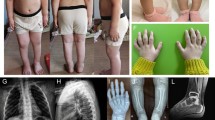
Ehlers-Danlos syndrome type IV, an inherited connective tissue disease, is usually caused by mutations in the gene for type III collagen. Here, we describe a glycine to glutamic acid substitution in a patient with this syndrome. Previous studies had shown that fibroblasts from the patient, his mother and brother secreted a reduced amount of type III collagen and also produced an overmodified form of the protein that was preferentially retained intracellularly. Peptide mapping experiments indicated that the mutation was located within cyanogen bromide peptide 9. This was supported by chemical cleavage analysis and sequencing of cDNA encoding this region. Allele-specific oligonucleotide hybridisation of genomic DNA confirmed that a G to A mutation converted Gly 847 to Glu. The mutation was present in two other affected family members and also in a third, who was clinically unaffected. Further analysis of this unaffected individual revealed reduced mutant:normal ratios in DNA obtained from both blood and hair samples, showing that she was mosaic for the mutation.
Similar content being viewed by others
References
Ala-Kokko L, Kontusaari S, Baldwin CT, Kuivaniemi H, Prockop DJ (1989) Structure of cDNA clones coding for the entire preproal(III) chain of human type III procollagen: differences in protein structure from type I procollagen and conservation of codon preferences. Biochem J 260:509–516
Bateman JF, Chan D, Mascara T, Rogers JG, Cole WG (1986) Collagen defects in lethal perinatal osteogenesis imperfecta. Biochem J 240:699–708
Beighton P (1970) The Ehlers-Danlos syndrome. William-Heineman, London
Bonadio J, Byers PH (1985) Subtle structural alterations in the chains of type I procollagen produce osteogenesis imperfecta type II. Nature 316:363–366
Breathnach AS, Smith J (1968) Fine structure of the early hair germ and dermal papilla in the human foetus. J Anat 102:511–526
Byers PH (1990) Brittle bones — fragile molecules:disorders of collagen gene structure and expression. Trends Genet 6:293–300
Cohn DH, Starman BJ, Blumberg B, Byers PH (1990) Recurrence of lethal osteogenesis imperfecta due to parental mosaicism for a dominant mutation in a human type I collagen gene (COL1A1). Am J Hum Genet 46:591–601
Cole WG, Chiodo AA, Lamande SR, Janeczko R, Ramirez F, Dahl HM, Chan D, Bateman JF (1990) A base substitution at a splice site in the COL3A1 gene causes exon skipping and generates abnormal type III procollagen in a patient with Ehlers-Danlos syndrome type IV. J Biol Chem 265:17070–17077
Cotton RGH, Rodrigues NR, Campbell RD (1988) Reactivity of cytosine and thymine in single base pair mismatches with hydroxylamine and osmium tetroxide and its application to the study of mutations. Proc Natl Acad Sci USA 85:4397–4401
Devereaux J, Haeberli P, Smithies O (1984) A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res 17:5961–5971
Fox R, Pope FM, Narcisi P, Nicholls AC, Kendall BE, Hourihan MD, Compston DAS (1988) Spontaneous carotid cavernous fistula in Ehlers-Danlos syndrome. J Neurol Neurosurg Psychiatry 51:984–986
Kontusaari S, Tromp G, Kuivaniemi H, Ladda RL, Prockop DJ (1990) Inheritance of an RNA splicing mutation (G+1IVS20) in the type III procollagen gene (COL3A1) in a family having aortic aneurysms and easy bruisability: phenotypic overlap between familial arterial aneurysms and Ehlers-Danlos syndrome type IV. Am J Hum Genet 47:112–120
Kuivaniemi H, Kontusaari S, Tromp G, Zhao M, Subol C, Prockop DJ (1990) Identical G+1 to A mutations in three different introns of the type III procollagen gene (COL3A1) produce different patterns of RNA splicing in three variants of Ehlers-Danlos syndrome IV. J Biol Chem 265:12067–12074
Kuivaniemi H, Tromp G, Prockop DJ (1991) Mutations in collagen genes. Causes of rare and some common diseases in man. FASEB J 5:2052–2060
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lee B, Vitale E, Superti-Furga A, Steinmann B, Ramirez F (1991a) G to T transversion at position +5 of a splice donor site causes skipping of the preceding exon in the type III procollagen transcripts of a patient with Ehlers-Danlos syndrome type IV. J Biol Chem 266:5256–5259
Lee B, D'Alessio M, Vissing H, Ramirez F, Steinmann B, Superti-Furga A (1991b) Characterisation of a large deletion associated with a polymorphic block of repeated dinucleotides in the type III procollagen gene (COL3A1) of a patient with Ehlers-Danlos syndrome type IV. Am J Hum Genet 48:511–517
Lench N, Stanier P, Williamson R (1988) Simple non-invasive method to obtain DNA for gene analysis. Lancet I:1356–1358
McKusick VA (1972) Heritable disorders of connective tissue, 4th edn. Mosby, St Louis, Mo, pp 292–371
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Nicholls AC, De Paepe A, Narcisi P, Dalgleish R, De Keyser F, Matton M, Pope FM (1988) Linkage of a polymorphic marker for the type III collagen gene (COL3A1) to atypical autosomal dominant Ehlers-Danlos syndrome type IV in a large Belgian pedigree. Hum Genet 78:276–281
Pope FM, Martin GR, Lichtenstein JR, Penttinen R, Gerson B, Rowe DW, McKusick VA (1975) Patients with Ehlers-Danlos syndrome type IV lack type III collagen. Proc Natl Acad Sci USA 72:1314–1316
Richards AJ, Lloyd JC, Ward PN, De Paepe A, Narcisi P, Pope FM (1991) Characterisation of a glycine to valine substitution at amino acid position 910 of the triple helical region of type III collagen in a patient with Ehlers-Danlos syndrome type IV. J Med Genet 28:458–463
Roberts RG, Montandon AJ, Bentley DR (1989) Detection of novel genetic markers by mismatch analysis. Nucleic Acids Res 17:5961–5971
Saiki RK, Scharf S, Faloona F, Mullis KB, Horn GT, Erlich HA, Arnheim N (1985) Enzymatic amplification of β-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 230:1350–1354
Sykes B, Puddle B, Francis M, Smith R (1976) The estimation of two collagens from human dermis by interrupted gel electrophoresis. Biochem Biophys Res Commun 72:1472–1480
Tromp G, Kuivaniemi H, Shikata H, Prockop DJ (1989a) A single base mutation that substitutes serine for glycine 790 of the α1(III) chain of type III procollagen exposes an arginine and causes Ehlers-Danlos syndrome IV. J Biol Chem 264:1349–1352
Tromp G, Kuivaniemi H, Stolle C, Pope FM, Prockop DJ (1989b) Single base mutation in the type III procollagen gene that converts the codon for glycine 883 to aspartate in a mild variant of Ehlers-Danlos syndrome IV. J Biol Chem 264:19313–19317
Tsipouras P, Byers PH, Schwartz RC, Chu M, Weil D, Pepe G, Cassidy SB, Ramirez F (1986) Ehlers-Danlos syndrome type IV: cosegregation of the phenotype to a COL3A1 allele of type III procollagen. Hum Genet 74:41–46
Vissing H, D'Alessio M, Lee B, Ramirez F, Byers PH, Steinmann B, Superti-Furga A (1991) Multiexon deletion in the procollagen III gene is associated with mild Ehlers-Danlos Syndrome type IV. J Biol Chem 266:5244–5248
Wallis GA, Starman BJ, Zinn AB, Byers PH (1990) Variable expression of osteogenesis imperfecta in a nuclear family is explained by somatic mosaicism for a lethal point mutation in the α1(I) gene (COL1A1) of type I collagen in a parent. Am J Hum Genet 46:1034–1040
Wood WI, Gitschier J, Lasky LA, Lawn RM (1985) Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci USA 82:1585–1588
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Richards, A.J., Ward, P.N., Narcisi, P. et al. A single base mutation in the gene for type III collagen (COL3A1) converts glycine 847 to glutamic acid in a family with Ehlers-Danlos syndrome type IV. An unaffected family member is mosaic for the mutation. Hum Genet 89, 414–418 (1992). https://doi.org/10.1007/BF00194313
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00194313